Abstract
The generalist predator Nesidiocoris tenuis, an effective biological control agent of numerous pests of the tomato crops, can also trigger plant defence mechanisms (direct and indirect) due to its phytophagous behaviour. In southern Europe, Nesidiocoris tenuis is frequently released in tomato greenhouses to control the invasive pest Tuta absoluta, sometimes combined with another biocontrol agent, the egg parasitoid Trichogramma achaeae. In this study, using olfactometer bioassays, we showed that the modification of the volatile chemical profile induced by the plant feeding activity of the mirid made tomato plants more attractive to T. achaeae, both in the absence and in the presence of T. absoluta eggs or larvae. This result was discussed in relation to difference observed among the chemical profiles of the volatile organic compounds released by tomato plants punctured by N. tenuis, without or in combination with T. absoluta infestation. We found that a few compounds (e.g. β-pinene and myrcene) could play a role in the foraging behaviour of T. achaeae in this scenario. Overall, our data support the emerging idea of an ecological role of N. tenuis, in addition to its predatory activity, in making tomato crops more resilient against the attack of pests, including T. absoluta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, predatory mirid bugs (Hemiptera: Miridae) have been increasingly used in tomato crops as biocontrol agents against a wide range of pests (i.e. aphids, whiteflies and lepidopterans) (van Lenteren 2012; Madeira et al. 2019; Pérez-Hedo et al. 2021; Ingegno et al. 2021). One of the most successful applications of these biocontrol agents is that of Nesidiocoris tenuis Reuter (Hemiptera: Miridae) released against the invasive South American tomato pinworm Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) (Chang and Metz 2021) in southern Europe (Urbaneja et al. 2012; Pérez-Hedo et al. 2017). In invaded regions, T. absoluta represents one of the most destructive pests of tomato and frequently requires repeated applications of synthetic insecticides to prevent economic damage (Giorgini et al. 2019; Desneux et al. 2022). Larvae of T. absoluta dig mines in tomato leaves and stems and burrow in fruits, causing injuries that lead to production losses of up to 80–100% (López 1991; Picanço et al. 1998). To date, more than 100 countries outside the native area of South America are facing this pest (EPPO 2022). Several biological and ecological aspects of this species have contributed to its spread and high impact on tomato crops (Sylla et al. 2017; Campos et al. 2021; de Campos et al. 2021; Ponti et al. 2021). These aspects include the high reproductive potential with multiple overlapping generations per year and high resistance to most used insecticides (Mansour et al. 2018; Cherif et al. 2019; Guedes et al. 2019). Therefore, several research projects focused on using biological control agents and biotechniques to reduce the synthetic inputs needed to preserve tomato production (Giorgini et al. 2019; Desneux et al. 2022). The use of N. tenuis has become a standard approach to control T. absoluta in wide areas of protected crops in southern Europe (Pérez-Hedo et al. 2017). However, other than feeding on arthropods, N. tenuis nymphs and adults can behave as herbivores by feeding on different plant parts (stem, sprouts, fruits and flower peduncles), causing reduced vegetative growth, necrotic rings and subsequent abortion of flowers and small fruits (Castañé et al. 2011; Calvo et al. 2012; Chinchilla-Ramírez et al. 2021; Pérez-Hedo et al. 2021). Consequently, when the mirid population is high and there is a shortage of arthropods to prey on, N. tenuis can become a pest (Arnó et al. 2010). The predator release must be properly managed, and sometimes low-impact insecticide sprayings are needed to regulate its populations and avoid economic losses (Pérez-Hedo and Urbaneja 2016; Pérez-Hedo et al. 2021). Currently, the omnivore N. tenuis, marketed in Europe, North Africa and Asia (Desneux et al. 2022), is mostly released in areas with warmer climates (i.e. Spain and South Italy) rather than in temperate zones (Pérez-Hedo and Urbaneja 2016). In southern Europe, the potential risk of tomato damage by N. tenuis is generally offset by its ability to control multiple key pests (e.g. whiteflies and T. absoluta) and to help reduce the populations of other pests (e.g. aphids, moths and thrips). Moreover, over the past decade, the mirids’ phytophagous behaviour has become an attractive added value for sustainable pest management programmes (Urbaneja et al. 2022). The feeding activity of N. tenuis on tomato plants induces direct and indirect defence mechanisms similar to the response exerted by these plants to harmful herbivores (Pérez-Hedo et al. 2022). In tomato plants, the injection of saliva by both nymphs and adults of N. tenuis (Naselli et al. 2016) triggers a cascade of events regulated by the activation of the jasmonic, abscisic and salicylic acid metabolic pathways (Pérez-Hedo et al. 2018a). Therefore, secondary metabolites, including VOCs and proteins, are specifically produced in the plant punctured by N. tenuis. These compounds have a role in direct plant defence being toxic, repellent and/or acting as antifeedants against herbivores, as well as in indirect defence through the attraction of natural enemies of herbivores (Pérez-Hedo et al. 2018b). For example the profile of VOCs emitted by tomato plants injured by N. tenuis includes three green leaf volatiles (GLVs) that are attractive to Encarsia formosa Gahan, a parasitoid of whitefly pests (Pérez-Hedo et al. 2018b). This evidence motivated us to investigate whether N. tenuis, by modifying the tomato plant VOC emissions, could have an impact on a biocontrol agent of T. absoluta, namely Trichogramma achaeae Nagaraja and Nagarkatti (Hymenoptera: Trichogrammatidae), an egg parasitoid of Lepidoptera (Polaszek et al. 2012). Inundative releases of T. achaeae have been considered an effective strategy to support T. absoluta management in tomato greenhouses of Mediterranean Countries (Giorgini et al. 2019; Desneux et al. 2022), either when the parasitoid is released alone (El-Arnaouty et al. 2014; Kortam et al. 2017) or in combination with predatory mirids (Chailleux et al. 2013; Cabello et al. 2015).
Our work starts from two recent findings: (i) feeding by N. tenuis adults and nymphs triggers volatile organic compounds (VOCs) by the injured plant that influences the behaviour of phytophagous pests and their natural enemies (Pérez-Hedo and Urbaneja 2016; Bouagga et al. 2018b; Pérez-Hedo et al. 2018b, 2021; Esmaeily et al. 2021); (ii) the parasitoid T. achaeae exploits VOCs emitted by tomato plants to locate its host (Gontijo et al. 2019). In this study, we try to answer the following questions: (i) does the phytophagy of N. tenuis influence the foraging behaviour of T. achaeae? (ii) does the phytophagy of N. tenuis alter the VOC blend released by un-infested or T. absoluta-infested tomato plants? and (iii) is the foraging behaviour of T. achaeae influenced by the contemporary presence of N. tenuis and T. absoluta on tomato plants?
Materials and methods
Plants and insects
The tomato plants (cultivar “San Marzano nano”) were grown in a glasshouse at 24 ± 2 °C, with relative humidity (RH) of 65 ± 5% and a photoperiod of 16L:8D h. Tuta absoluta was reared on caged tomato plants in the same conditions starting from adult moths collected during the summer of 2019 in tomato greenhouses in Battipaglia (Salerno, Italy). Plants and T. absoluta colonies were maintained at the Istituto per la Protezione Sostenibile delle Piante, Portici, Italy.
The parasitoid T. achaeae was purchased biweekly from a commercial supplier (Agrobio, Almeria, Spain) as pupae developed in the eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Upon arrival, they were placed under the same climatic conditions described above. The identity of the Trichogramma species was confirmed by COI and ITS2 gene amplification and sequencing performed on ten individuals randomly selected, as described by Cascone et al. (2015). Female parasitoids used in olfactometer tests were 24-h old, mated, fed (1:1 water/honey solution) and naïve, i.e. with no previous oviposition experience or contact with plants or host instars. Adult parasitoids were placed individually in micro-glass vials and sexed under a stereoscope 1–2 h prior to olfactometer tests.
Adults of the predator N. tenuis were purchased biweekly from a commercial supplier (Agrimpol, Montecorvino Pugliano, Italy). They were reared on tomato plants in 35 × 35 × 60-cm mesh cages, in a climate-controlled glasshouse at 24 ± 2 °C, 65 ± 5% RH and 16:8 (L:D) h photoperiod. Adult mirids were fed with eggs of E. kuehniella (purchased from Bioplanet, Cesena, Italy) ad libitum and ~100 aphids (Macrosiphum euphorbiae L.) infesting the tomato plants.
Olfactometer bioassay
The tomato plants used in the behavioural assay were 5 weeks old, with 4–6 fully expanded leaves and 18–20-cm tall. The behavioural responses of T. achaeae females to plant volatiles were measured and investigated in a Y-tube olfactometer. The latter consisted of a 1-cm diameter Y-shaped glass tube with a 9-cm long base and two 8-cm long arms connected to two glass jars as described in detail by Cascone et al. (2019). Tomato plants to be tested were placed individually inside one of the 20-L glass jars. The jars were closed tightly for 15–20 min prior to the beginning of each choice test to allow the sufficient diffusion of odour plumes and the achievement of a pressure balance inside the system. Each of the two glass jars was independently connected to the two arms of the Y-tube, and the airflow was adjusted to 100 ml/min for each arm. All tests were conducted between 10:00 and 14:00 h in a laboratory evenly illuminated by fluorescent lights (PPFD of 700 μmol m2s−1) at 24 ± 2 °C. Olfactometer bioassays were performed by testing multiple female wasps simultaneously (Gols et al. 2012; Cusumano et al. 2015). A preliminary bioassay was done to exclude the possibility of a grouping effect. Ten groups of ten female wasps each were tested in an olfactometer two-choice bioassay towards the same odour source (a tomato plant infested by T. absoluta eggs) to verify whether the tested females would affect each other’s choice when released in a group. The wasps’ distribution in the two arms of the Y-tube olfactometer (46 versus 54) did not significantly differ from 50:50 (two-sided binomial test, P = 0.484). Thus, all subsequent experiments were performed releasing wasps in groups. Ten wasp females were released into the system simultaneously, and two groups of females, representing a single experimental replicate for each plant pair tested, were observed per tested plant pair (Cascone et al. 2019; Gontijo et al. 2019). The position of the plants was swapped after having tested the first group of ten females to avoid any position bias. The choice between the two odour sources (tested plants) was recorded within 30 min from the parasitoid release, with a choice considered to be made when wasps were found inside the trapping bulbs, located near the ends of the Y-tube arms. Wasps that did not respond (overall average 22.6 ± 1.2% SE) within the observation period were scored as non-responding and were excluded from the statistical analysis. Female parasitoids were tested against the following odour sources: clean air (A), healthy tomato plants (H), N. tenuis punctured plants (N), N. tenuis punctured plants + T. absoluta eggs (N + TE) and N. tenuis punctured plants + T. absoluta larvae (N + TL). The odour sources were tested in nine combinations: (1) N + TE versus N + TL, (2) N versus N + TL, (3) N versus N + TE, (4) H versus N + TL, (5) A versus N + TL, (6) H versus N + TE, (7) A versus N + TE, (8) H versus N and (9) A versus N. Each combination was replicated ten times over 10 different days using different plants. Overall, 200 T. achaeae females were tested for each bioassay combination. The total number of T. achaeae attracted to one of the two odour sources (attractiveness), and the total number of wasps that made a choice (responsiveness) were subjected to a binomial test to assess if the observed distribution differed significantly from an equal distribution (1:1) between the two odour sources. Attractiveness and responsiveness were used as “number of successes” while wasps making a choice and total wasps tested as “number of trials”, respectively. Analyses were made using the function “binom.test” within the R statistics programming environment 3.3.3 (R Core Team 2022). Olfactometer bioassays were run at the Istituto per la Protezione Sostenibile delle Piante, Portici, Italy.
Plant treatments
All experimental treatments are summarized in Fig. 1.
Representation of the time sequence of plant infestation events for each of the three experimental treatments. Nesidiocoris tenuis punctured (N) tomato plants were obtained by exposing them to N. tenuis adults for 24 h. Nesidiocoris tenuis punctured + Tuta absoluta eggs (N + TE) plants were obtained by exposing them to mirids and after to T. absoluta adults for 24–48 h. Nesidiocoris tenuis punctured + T. absoluta larvae (N + TL) plants were exposed first to T. absoluta adults for 24–48 h, then reared for 7–10 days to allow the eggs hatching into larvae and eventually exposed to mirids adults for 24 h
Nesidiocoris tenuis punctured plants (N) were obtained by allowing 100 mirids to feed on four healthy plants (in average 25 mirids per plant) placed in a cage for 24 h, after that the insects were removed, and the plant stored in a new cage prior to test it in olfactometer. For the N + TE treatment, N. tenuis punctured plants (as described above) were immediately subjected to T. absoluta oviposition for 48–72 h prior to olfactometer tests. This exposure time resulted in N + TE plants with an average of 26.50 ± 3.00 (mean ± SE) T. absoluta eggs. For the N + TL treatment, plants were first exposed to T. absoluta ovipositing females for 24–48 h, then kept within cages for 7–10 days to allow the eggs hatching into larvae. Plants bearing an average of 10.30 ± 1.30 (mean ± SE) T. absoluta first instar larvae were produced. Finally, these plants were exposed to N. tenuis adults for 24 h as described above for the N treatment.
VOCs collection and analysis
Soon after the olfactometer bioassay, glass jars containing the tomato plants were connected to an airtight entrainment system consisting of a circulating pump (closed-loop) whose flow was adjusted to 200 cm3 min−1 for the collection of VOCs from the headspace. Before re-entering the pump, the air passed through an adsorbent cartridge made of a narrow glass tube filled with a biphasic phase of 30 mg of Tenax and 30 mg of Carboxen. Ten tomato plants for each treatment were sampled individually for 3 h to collect volatiles. Volatile cartridges were analysed by CIS4–TDU–GC/MS. Gerstel TDU heated at 300 °C for 7 min under a helium stripping flow of 30 ml min−1. The TDU unit was directly assembled to the PTV injector (CIS4 Gerstel, Germany) with a liner-in-liner coupling, eliminating the carry-over effect and analyte loss. During this process, the CIS4 was cooled to −20 °C by computer-controlled liquid CO2 pulsed flow. After cryo-trapping on a Tenax packing liner, the PTV was quickly ramped to 260 °C for desorption, and the analyte was transferred to CIS4. Agilent® 7890 GC equipped with an Agilent ® 5975 MSD (Palo Alto, USA) was used for the analysis with helium chosen as carrier gas kept at a constant flow of 1.2 ml min −1. The chromatographic settings were as follows: injector in splitless mode set at 260 °C, J&W Innowax column (50 m, 0.20 mm i.d., 0.4 um df); oven temperature programme: initial temperature 40 °C for 1 min, then 10 °C min−1 increase until 130 °C, then 5 °C min−1 increase until 210 °C, then 20 °C min−1 increase until 260 °C, hold time 3 min. The mass spectrometer operated with an electron ionization of 70 eV, in scan mode in the m/z range 29–330, at three scans sec−1. The deconvoluted peaks spectra obtained by Agilent MassHunter® software were matched against the NIST 11 spectral library for tentative identification. Kovats’ retention indices were calculated for further compound confirmation and compared with those reported in the literature for the chromatographic column used (Kovat 1965). Authentic standards were also injected into the system to confirm the tentative identity of the compounds. The volatile emission patterns were standardized by leaf surface measured by Easy Leaf Area software (Easlon and Bloom 2014) on all leaves from each tested plant. A one-way ANOVA analysis was run to assess if the quantity of each VOC emitted by tomato plants differed among the four treatments. To verify if data met the ANOVA assumptions, the Shapiro–Wilk’s test and Q–Q plots were used to check for normality and the Levene’s test for homogeneity of variances of each individual VOC. When ANOVA assumptions were not satisfied, log transformation achieved normality and homogeneity of variances for the dependent variable. When the ANOVA test was significant, Tukey’s HSD post hoc test at the confidence level of 0.95 was conducted to test which means are different in pairwise comparisons of plant treatment. When the assumption of equal variances was violated, even after data transformation, comparisons were made using the non-parametric Kruskal–Wallis test followed by the Fisher's least significant difference test using the R package “Agricolae” (de Mendiburu 2021). Multivariate analysis of variance (MANOVA) and linear discriminant analysis (LDA) were then applied to explore the possibility of differentiating VOC profiles according to mirids phytophagy and T. absoluta infestation using the R package “MASS” (Venables and Ripley 2002). Chemical analyses were run at the Portici and Firenze units of the Istituto per la Protezione Sostenibile delle Piante.
Results
Nesidiocoris tenuis feeding triggers Trichogramma achaeae host searching
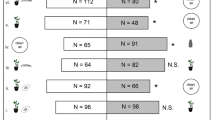
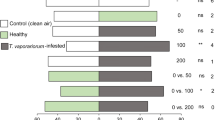
Plants punctured by N. tenuis (N, N + TE and N + TL) were more attractive towards T. achaeae females than clean air or healthy (H) plants (Fig. 2a).
Results of the two-choice olfactometer bioassays. (a) Preference (percentage) of Trichogramma achaeae female wasps in a two-choice setup in a Y-tube olfactometer. Different combinations of clean air (A), healthy (H), Nesidiocoris tenuis punctured (N), N. tenuis punctured + Tuta absoluta eggs (N + TE) and N. tenuis punctured + T. absoluta larvae (N + TL) tomato plants were tested. A number of female parasitoids that have chosen the two odour sources are reported in parentheses. (b) Percentage and number (in parenthesis) of T. achaeae female wasps who made a choice. Asterisks indicate significant statistical differences between the percentages of parasitoids choosing one of the two odour sources or between wasps making or not a choice (binomial test, *P < 0.05, ***P < 0.001). ns means no significant difference
The combination of N. tenuis punctures and T. absoluta larval feeding (N + TL) was more attractive to T. achaeae than both N treatment (tomato plants punctured by N. tenuis) and N + TE treatment (tomato plants with N. tenuis punctures in combination with T. absoluta eggs) (Fig. 2a). Parasitoid females did not make a choice between N and N + TE treatments (Fig. 2a). Overall, the attractiveness was high and statistically significant in all olfactometer bioassays with an average of 76.3% ± 1.3% SE of wasps making a choice (Fig. 2b).
Nesidiocoris tenuis feeding modifies VOC emission by tomato plants
Nesidiocoris tenuis punctures alone (N) and in combination with T. absoluta oviposition (N + TE) or larval feeding (N + TL) significantly enhanced the total emissions of VOCs by tomato plants (MANOVA, Pillai’s trace = 2.320, F39,78 = 3.563, P < 0.001). LDA on plants from the different treatments succeeded in splitting them into appropriate groups, with the first two functions explaining 98% of the total variance (Fig. 3). In total, 13 plant-related volatile compounds were identified from the headspace of all treatments (Fig. 4).
Linear discriminant analysis (LDA) of VOCs produced by tomato plants. Each point represents the multivariate VOC profile of a single plant in LDA space, belonging to one of the four treatments (H = healthy, N = Nesidiocoris tenuis punctured, N + TE = N. tenuis punctured + Tuta absoluta eggs and N + TL = N. tenuis punctured + T. absoluta larvae). Black dots represent the centroids. Dotted lines represent 95% confidence interval ellipses for each group
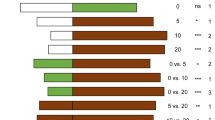
VOCs emitted by tomato plants. Histograms represent the mean amounts and the bars the standard error of the VOCs emitted by healthy (H), punctured by N. tenuis (N), punctured by N. tenuis and infested by T. absoluta eggs (N + TE) and punctured by N. tenuis and infested by T. absoluta larvae (N + TL) tomato plants. For each VOC, different letters mean a significant difference in pairwise comparisons (Tukey’s HDS post hoc test or Kruskal–Wallis test, P < 0.05)
All compounds except camphene, β-pinene and sabinene were released at a significant higher rate by plants exposed to the feeding activity of N. tenuis (N) in respect to healthy plants (H) (Fig. 4). Similarly, both eggs oviposition (N + TE) and larval feeding by T. absoluta (N + TL) triggered a significant increase in the emission of almost all compounds in respect to healthy plants (H). Exceptions are represented by α-terpinene, β-phellandrene and carvacrol, which were not influenced by N + TE treatment in respect to healthy plants (H). The emission of α-terpinene was not influenced by either N + TE or N + TL treatments in respect to control (H) plants. The activity of T. absoluta (eggs or larvae) combined to mirids phytophagy (N + TE or N + TL) resulted in the significant lower emission of 2-carene, myrcene, ɑ-phellandrene, ɑ-terpinene, limonene, β-phellandrene, p-cymene and carvacrol in respect to only punctured (N) plants. On the contrary, the release of ɑ-pinene, camphene, β-pinene, sabinene and methyl salicylate was significantly higher in plants infested by T. absoluta (N + TE or N + TL) in respect to only punctured plants (N). Methyl salicylate was the most abundant compound found in the VOC blends of all treatments (Fig. 4). Camphene, β-pinene and sabinene were mostly emitted by tomato plants infested by T. absoluta eggs or larvae, while p-cymene and methyl salicylate were mostly emitted by tomato plants exposed to mirids, with or without T. absoluta (Fig. 4).
Discussion
The omnivore N. tenuis, marketed in Europe, North Africa and Asia (Desneux et al. 2022), is commonly released into greenhouses to control the invasive pest T. absoluta infesting tomato crops. In addition to its direct activity as predator of insect pests, seasonal releases of N. tenuis are now conceived as a mean to increase the resilience of greenhouse crops (Urbaneja et al. 2022). Indeed, when N. tenuis feeds on host plants, it induces the activation of direct and indirect defence mechanisms similar to the response exerted by these plants to harmful herbivores (Pérez-Hedo et al. 2022), thus increasing the ability of the host plants to deal with pest attack. For example sweet pepper and tomato plants eaten upon by N. tenuis, can repel Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) and Tetranychus urticae Koch (Acari: Tetranychidae) (Pérez-Hedo et al. 2015, 2018a, b; Bouagga et al. 2018b, a), while being attractive to the whitefly parasitoid Encarsia formosa Gahan (Hymenoptera: Aphelinidae) (Pérez-Hedo et al. 2015; Bouagga et al. 2018a). In this paper, we have deepened the knowledge on the role played by N. tenuis in the regulation of multitrophic interactions of tomato plants by focusing on the impact of the predator, alone or in combination with the pest T. absoluta, on the foraging behaviour of the egg parasitoid T. achaeae. The interest about this parasitoid species is motivated by its use as a biological control agent of T. absoluta by inundative releases into tomato greenhouses, even in combination with predatory mirids (Chailleux et al. 2013; Cabello et al. 2015; El-Arnaouty et al. 2014; Kortam et al. 2017). It must be considered, however, that the inundative release of T. achaeae is in general an expensive strategy (Desneux et al. 2022), not always economically sustainable for the tomato crops. To face this problem, recent research aimed to exploit olfactory cues related to the T. absoluta—tomato plant complex to increase the searching behaviour and the parasitization efficiency of T. achaeae (Gonthier et al. 2022). However, both Gonthier et al. (2022) and Milonas et al. (2019) did not find any attraction of the parasitoid to tomato plants infested by T. absoluta eggs in olfactometer bioassay. Conversely, our group (Gontijo et al. 2019) highlighted the innate capacity of T. achaeae to exploit VOCs emitted by tomato plants, regardless of the presence of T. absoluta larvae and eggs, to locate its host. These conflicting results can be explained by the different tomato cultivars used in the three studies and by the fact that in Gonthier et al. (2022), the olfactometer bioassays were conducted with leaves excised by the plant and using a different experimental setup. For example it has been demonstrated that leaf cutting induces the emission of VOCs and activates a wound-eliciting mechanism (Rasulov et al. 2019 and reference therein). Hence, using cut leaves can affect the characterization of VOCs released upon herbivore attack and their subsequent effect on the natural enemies’ behaviour. In the present study, performed using the same tomato cultivar tested in our previous works, the plants eaten by N. tenuis (N), even when infested by T. absoluta eggs (N + TE) or larvae (N + TL), resulted more attractive to T. achaeae than healthy plants (H). In addition, the fact that in this study, the egg parasitoid was significantly more attracted to plants injured by N. tenuis than to clean air further confirms the results obtained by Gontijo et al. (2019). Here, we showed that N. tenuis feeding activity helped the egg parasitoid in locating its host more efficiently. This result was supported by the different VOC profiles produced by tomato plants punctured by N. tenuis compared to healthy plants. All compounds, with the exception of carvacrol, are emitted in greater amounts by the plants infested by T. absoluta (N + TE and N + TL) (Fig. 4), which is in agreement with the results of the previous studies on tomato plant infested by T. absoluta eggs (Anastasaki et al. 2015; Naselli et al. 2017; Gontijo et al. 2019; Milonas et al. 2019) or larvae (De Backer et al. 2015; Naselli et al. 2017; Silva et al. 2017; Anastasaki et al. 2018; Gontijo et al. 2019; Abdollahipour et al. 2020; Ayelo et al. 2021, 2022; Maneesha et al. 2021; Agbessenou et al. 2022). However, the VOCs emitted by N. tenuis punctured plants in this study only partially overlap with those found by Pérez-Hedo et al. (2018b). The latter showed that the VOC profile of tomato plants injured by N. tenuis differed from that of healthy plants by significantly greater quantities of methyl salicylate and three green leaf volatiles ((Z)-3-hexenyl butanoate, (Z)-3-hexenyl propanoate and (Z)-3-hexenol). In N. tenuis punctured plants, we detected larger amounts only of two of these compounds, namely methyl salicylate (Fig. 4) and trace amounts of 3-hexenol (data not shown). The differences between the two studies can be explained by the major differences in the methods used to collect the volatiles. The comparison between SPME (used by Pérez-Hedo et al. 2018b) and the adsorption on Tenax followed by thermal desorption (here adopted) revealed dramatic variations in the volatile profile obtained by tomato plants (see Rambla et al. 2015). These two techniques differ in their ability to capture VOCs with different vapour pressures (Rambla et al. 2015). SPME fibres are effective for analysing VOCs with low volatility, whereas Tenax cartridges are better suited for VOCs with high volatility. This is in line with the results of our study where compounds such as α-pinene and sabinene, which have high volatility (vapour pressures of 4.75 and 2.6 mmHg at 25 °C (PubChem), respectively), were detected using Tenax cartridges, while compounds such as octyl acetate and hexyl butanoate, which have lower volatility (vapour pressures of 0.4 and 0.2 mmHg at 25 °C (PubChem), respectively), were not detected. The latter compounds were instead recorded by Pérez-Hedo et al. 2018b using SPME fibres. Moreover, even the tomato cultivar used could be responsible for the differences recorded. Here, we tested the cultivar “San Marzano nano”, characterized by a determinate growth habit while other authors used the cultivar “Optima”, characterized by an indeterminate growth habit. The genetic background, specific for each cultivar, regulates both the quantity and quality of volatiles emitted (Raghava et al. 2010). However, since we detected VOCs emitted by tomato plants in response to N. tenuis that were not detected by Pérez-Hedo et al. (2018b), the combined results of the two studies help to better understand the chemical response of tomato plants to N. tenuis.
Methyl salicylate was found to be emitted by plants in response to both T. absoluta infestation (Silva et al. 2017; Anastasaki et al. 2018; Gontijo et al. 2019; Milonas et al. 2019; Ayelo et al. 2021, 2022; Agbessenou et al. 2022) and N. tenuis punctures (Pérez-Hedo et al. 2018b). Here, we recorded an additive effect due to T. absoluta and N. tenuis with significant increases in N + TE and N + TL treatments compared to the control N treatment (Fig. 4). However, it is reasonable to hypothesize that other VOCs can be induced specifically by N. tenuis damage in tomato plants. For example carvacrol has never been identified in the VOC profiles released by tomato plants infested by T. absoluta eggs (Anastasaki et al. 2015; Naselli et al. 2017; Gontijo et al. 2019; Milonas et al. 2019) or larvae (De Backer et al. 2015; Naselli et al. 2017; Silva et al. 2017; Anastasaki et al. 2018; Gontijo et al. 2019; Abdollahipour et al. 2020; Ayelo et al. 2021, 2022; Maneesha et al. 2021; Agbessenou et al. 2022). Here, we recorded the presence of carvacrol in all N. tenuis treatments (N, N + TE and N + TL). The compound myrcene also seems to be produced by tomato plants in response to N. tenuis feeding. Indeed, it was not found to be associated with T. absoluta oviposition alone (Anastasaki et al. 2015; Naselli et al. 2017; Gontijo et al. 2019; Milonas et al. 2019), and here, we reported a consistent release in all N. tenuis treatments (N, N + TE and N + TL).
All VOCs identified in our study (with the exception of camphene, limonene and carvacrol) have been previously recorded in the headspace of tomato plants infested by T. absoluta and tested for their capacity to induce an electro-antennographic response of T. achaeae (Milonas et al. 2019). The authors showed that parasitoid females responded mostly to minor compounds rather than major compounds emitted by T. absoluta-infested plants. Small qualitative differences and specific ratios are usually more important than obvious quantitative differences in volatiles that affect insect behaviour (Bruce et al. 2010). Coherently, terpenes such as β-pinene and myrcene, produced in small quantities by tomato plants (Fig. 4) and found to be electroantennographically active compounds for T. achaeae (Milonas et al. 2019), could explain our results. To better explain this concept, we have summarized behavioural bioassay results (Fig. 2) and β-pinene and myrcene emissions by tomato plants (Fig. 4) in a supplementary figure (Fig. S1). When we tested plants fed upon by N. tenuis (N) against healthy plants (H), the parasitoid showed a preference for N plants which were characterized by a higher release of myrcene (Fig. S1). Similarly, both N + TE and N + TL plants emitted greater amounts of myrcene and β-pinene in respect to the healthy ones (H) (Fig. S1). The two compounds could be responsible for the attractiveness of these treatments when tested against the healthy plants. The emission of these two compounds could also explain the attractiveness observed in the choice tests N versus N + TL, N + TE versus N + TL and N versus N + TE (Fig. S1). In fact, mirids activity in combination with larvae induced a significantly higher emission of myrcene and β-pinene and resulted more attractive towards the parasitoid in respect to N. tenuis punctured plants (Fig. S1). The link between VOCs and attractiveness was registered also for myrcene in the bioassay N + TE versus N + TL (Fig. S1). On the contrary, the emission of myrcene and β-pinene had opposite trends for the bioassay N versus N + TE. In fact, mirids phytophagy and T. absoluta eggs infestation (N + TE) emitted lower amount of myrcene than N. tenuis punctured (N) plants (Fig. S1), while N + TE plants emitted a greater amount of β-pinene than N plants (Fig. 4). These contrasting values in VOC emissions can explain the non-preference of T. achaeae in the choice test N versus N + TE and support the idea that myrcene and β-pinene could have a pivotal role in the attraction of T. achaeae females. Moreover, in our experiment, the VOCs camphene, limonene and carvacrol showed emission patterns and significant differences between the plant treatments (H, N, N + TE and N + TL) similar to myrcene and β-pinene. Limonene and carvacrol resulted to be electrophysiologically active for the egg parasitoid Trichogramma chilonis Ishii (Sen et al. 2005). Measuring the EAD responses of T. achaeae females to camphene, limonene and carvacrol, which were not found and tested by Milonas et al. (2019), may help to better clarify the parasitoid behaviour observed in our bioassay. Finally, it should be pointed out that the differential attractiveness of the four plant treatments towards T. achaeae females was also supported by LDA analyses that separated the N, N + TE and N + TL tomato plants from the healthy plants (H) on the basis of their VOCs profiles.
The inundative release of the egg parasitoid T. achaeae in combination with the inoculative release of the predator N. tenuis has been suggested as a reliable strategy to increase the effectiveness of biological control of T. absoluta in southern Europe (Cabello et al. 2012, 2015). We found that the feeding activity of N. tenuis on tomato plants does not represent a factor hampering the attraction of T. achaeae to tomato plants. In fact, the egg parasitoid was preferentially attracted towards tomato plants injured by N. tenuis, either in the absence or in the presence of T. absoluta eggs or larvae, compared to healthy plants. Considering that N. tenuis is usually released in tomato greenhouses at the beginning of the cultivation cycle, even before T. absoluta arrives on the crop (Desneux et al. 2022), our results suggest that T. achaeae could be released both at the beginning of the infestation of T. absoluta, when the moth eggs are prevalent, and at medium–high levels of infestation during the crop cycle to improve the control activity of the predator alone (Cabello et al. 2015). The delayed release of T. achaeae should also avoid any negative effect, observed for a different Trichogramma species and associated with a shortage of moth eggs, consisting in the reduction of the efficacy of N. tenuis and in the higher number of plants injured by predator phytophagy (Mirhosseini et al. 2019). Even if more studies are needed to measure the effect of intraguild predation by N. tenuis and T. absoluta eggs parasitized by T. achaeae, we have highlighted that the mirid enhances the parasitoids attractiveness. This suggests a complementary action of both species where the IPM strategy relies on conservation of both N. tenuis and T. achaeae populations (Desneux et al. 2022). In conclusion, our results further confirm: The possibility of integrating the inundative release of T. achaeae in a sustainable IPM strategy aimed at the maximum possible reduction of synthetic insecticides; the ecological role of N. tenuis in increasing the resilience of tomato plants to biotic stressors (Pérez-Hedo et al. 2022).
Together with the results of Pérez-Hedo et al. (2018a, b), our findings suggest that indirect defence mechanisms activated by N. tenuis can be exploited by multiple natural enemies of different pests benefiting IPM strategies that rely on the augmentation or conservation of the natural enemies.
Author contributions
MG, EG, PC and PS designed the experiments; FT and PC performed bioassays; PC and MG supervised the experimental work; GC and MM performed VOC analysis and PC performed the statistical analysis. PC wrote the manuscript; EG and MG revised the manuscript. All the authors contributed to the manuscript writing.
Availability of data and materials
The datasets of VOC emitted by plants and of olfactometer bioassays supporting the conclusions of this article are available from the corresponding author on reasonable request.
Change history
02 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10340-023-01676-8
References
Abdollahipour M, Fathipour Y, Mollahosseini A (2020) How does a predator find its prey? Nesidiocoris tenuis is able to detect Tuta absoluta by HIPVs. J Asia-Pac Entomol 23:1272–1278. https://doi.org/10.1016/j.aspen.2020.10.006
Agbessenou A, Akutse KS, Yusuf AA, Khamis FM (2022) The endophyte Trichoderma asperellum M2RT4 induces the systemic release of methyl salicylate and (Z)-jasmone in tomato plant affecting host location and herbivory of Tuta absoluta. Front Plant Sci 13:860309. https://doi.org/10.3389/fpls.2022.860309
Anastasaki E, Balayannis G, Papanikolaou NE et al (2015) Oviposition induced volatiles in tomato plants. Phytochem Lett 13:262–266. https://doi.org/10.1016/j.phytol.2015.07.007
Anastasaki E, Drizou F, Milonas PG (2018) Electrophysiological and oviposition responses of Tuta absoluta females to herbivore-induced volatiles in tomato plants. J Chem Ecol. https://doi.org/10.1007/s10886-018-0929-1
Arnó J, Castañé C, Riudavets J, Gabarra R (2010) Risk of damage to tomato crops by the generalist zoophytophagous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). Bull Entomol Res 100:105–115. https://doi.org/10.1017/S0007485309006841
Ayelo PM, Yusuf AA, Pirk CWW et al (2021) Terpenes from herbivore-induced tomato plant volatiles attract Nesidiocoris tenuis (Hemiptera: Miridae), a predator of major tomato pests. Pest Manag Sci. https://doi.org/10.1002/ps.6568
Ayelo PM, Mohamed SA, Chailleux A et al (2022) The parasitoid Dolichogenidea gelechiidivoris eavesdrops on semiochemicals from its host Tuta absoluta and tomato. J Pest Sci 95:633–652. https://doi.org/10.1007/s10340-021-01424-w
Bouagga S, Urbaneja A, Pérez-Hedo M (2018a) Combined use of predatory mirids with Amblyseius swirskii (Acari: Phytoseiidae) to enhance pest management in sweet pepper. J Econ Entomol 111:1112–1120
Bouagga S, Urbaneja A, Rambla JL et al (2018b) Zoophytophagous mirids provide pest control by inducing direct defences, antixenosis and attraction to parasitoids in sweet pepper plants. Pest Manag Sci 74:1286–1296
Bruce TJA, Midega CAO, Birkett MA et al (2010) Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol Lett 6:314–317. https://doi.org/10.1098/rsbl.2009.0953
Cabello T, Gallego JR, Fernandez FJ et al (2012) Biological control strategies for the South American tomato moth (Lepidoptera: Gelechiidae) in greenhouse tomatoes. J Econ Entomol 105:2085–2096. https://doi.org/10.1603/ec12221
Cabello T, Bonfil F, Gallego JR et al (2015) Can interactions between an omnivorous hemipteran and an egg parasitoid limit the level of biological control for the tomato pinworm? Environ Entomol 44:12–26
Calvo FJ, Lorente MJ, Stansly PA, Belda JE (2012) Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol Exp Appl 143:111–119. https://doi.org/10.1111/j.1570-7458.2012.01238.x
Campos MR, Amiens-Desneux E, Béarez P et al (2021) Impact of low temperature and host plant on Tuta absoluta. Entomol Exp Appl 169:984–996. https://doi.org/10.1111/eea.13094
Cascone P, Carpenito S, Slotsbo S et al (2015) Improving the efficiency of Trichogramma achaeae to control Tuta absoluta. Biocontrol 60:761–771. https://doi.org/10.1007/s10526-015-9684-1
Cascone P, Gols R, Fatouros NE et al (2019) The effect of rearing history and aphid density on volatile-mediated foraging behaviour of Diaeretiella rapae. Ecol Entomol. https://doi.org/10.1111/een.12704
Castañé C, Arnó J, Gabarra R, Alomar O (2011) Plant damage to vegetable crops by zoophytophagous mirid predators. Biol Control 59:22–29
Chang PEC, Metz MA (2021) Classification of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae: Gelechiinae: Gnorimoschemini) based on cladistic analysis of morphology. Proc Entomol Soc Wash 123:41–54
Cherif A, Attia-Barhoumi S, Mansour R et al (2019) Elucidating key biological parameters of Tuta absoluta on different host plants and under various temperature and relative humidity regimes. Entomol Generalis 39:1–7
Chinchilla-Ramírez M, Garzo E, Fereres A et al (2021) Plant feeding by Nesidiocoris tenuis: quantifying its behavioral and mechanical components. Biol Control 152:104402. https://doi.org/10.1016/j.biocontrol.2020.104402
Cusumano A, Weldegergis BT, Colazza S et al (2015) Attraction of egg-killing parasitoids toward induced plant volatiles in a multi-herbivore context. Oecologia 179:163–174. https://doi.org/10.1007/s00442-015-3325-3
De Backer L, Megido RC, Fauconnier M-L et al (2015) Tuta absoluta-induced plant volatiles: attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod-Plant Interact 9:465–476. https://doi.org/10.1007/s11829-015-9388-6
de Mendiburu F (2021) Agricolae: statistical procedures for agricultural research. R Package Version 1(3–5):155
de Campos MR, Béarez P, Amiens-Desneux E et al (2021) Thermal biology of Tuta absoluta: demographic parameters and facultative diapause. J Pest Sci 94:829–842. https://doi.org/10.1007/s10340-020-01286-8
Desneux N, Han P, Mansour R et al (2022) Integrated pest management of Tuta absoluta: practical implementations across different world regions. J Pest Sci 95:17–39. https://doi.org/10.1007/s10340-021-01442-8
Easlon HM, Bloom AJ (2014) Easy leaf area: automated digital image analysis for rapid and accurate measurement of leaf area. Appl Plant Sci 2:1400033
El-Arnaouty S, Galal H, Afifi A et al (2014) Assessment of two Trichogramma species for the control of Tuta absoluta in North African tomato greenhouses. Afr Entomol 22:801–809
EPPO (2022) https://gd.eppo.int/taxon/GNORAB/distribution. Accessed 9 Mar 2022
Esmaeily S, Samih MA, Izadi H (2021) Induced resistance by jasmonic and abscisic acids and Nesidiocoris tenuis feeding on Solanum lycopersicum against Trialeurodes vaporariorum. Int J Pest Manag 67:46–57. https://doi.org/10.1080/09670874.2019.1669843
Giorgini M, Guerrieri E, Cascone P, Gontijo L (2019) Current strategies and future outlook for managing the neotropical tomato pest Tuta absoluta (Meyrick) in the Mediterranean basin. Neotrop Entomol. https://doi.org/10.1007/s13744-018-0636-1
Gols R, Huigens ME, Weldegergis BT et al (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7:e43607. https://doi.org/10.1371/journal.pone.0043607
Gonthier J, Zhang Y-B, Zhang G-F et al (2022) Odor learning improves efficacy of egg parasitoids as biocontrol agents against Tuta absoluta. J Pest Sci. https://doi.org/10.1007/s10340-022-01484-6
Gontijo L, Cascone P, Giorgini M et al (2019) Relative importance of host and plant semiochemicals in the foraging behavior of Trichogramma achaeae, an egg parasitoid of Tuta absoluta. J Pest Sci. https://doi.org/10.1007/s10340-019-01091-y
Guedes R, Roditakis E, Campos M et al (2019) Insecticide resistance in the tomato pinworm Tuta absoluta: patterns, spread, mechanisms, management and outlook. J Pest Sci 92:1329–1342
Ingegno BL, Messelink GJ, Leman A et al (2021) Development and thermal activity thresholds of European mirid predatory bugs. Biol Control 152:104423. https://doi.org/10.1016/j.biocontrol.2020.104423
Kortam MN, El Arnaouty S, Fatnassi H et al (2017) The effect of microclimatic parameters on two Trichogramma species used to control Tuta absoluta. IOBC-WPRS Bull 124:137
Kovat E (1965) The retention index system. Advances in chromatography. Marcel Dekker Inc, New York, pp 229–247
López E (1991) Polilla del tomate: Problema crítico para la rentabilidad del cultivo de verano. Empresa y Avance Agrícola 1:6–7
Madeira F, Edo E, Sossai S et al (2019) Pre-planting inoculation for early establishment of Dicyphus bolivari and D. errans on tomatoes. Biocontrol 64:33–41. https://doi.org/10.1007/s10526-018-09911-3
Maneesha A, Rao SRK, Bakthavatsalam N (2021) Behavioural mechanism of Tuta absoluta towards conspecific-heterospecific infested tomato plants in response to leaf volatiles. J Entomol Zool Stud 9:2053–2058
Mansour R, Brévault T, Chailleux A et al (2018) Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomol Generalis 38:83–112. https://doi.org/10.1127/entomologia/2018/0749
Milonas GP, Anastasaki E, Partsinevelos G (2019) Oviposition-induced volatiles affect electrophysiological and behavioral responses of egg parasitoids. InSects. https://doi.org/10.3390/insects10120437
Naselli M, Urbaneja A, Siscaro G et al (2016) Stage-related defense response induction in tomato plants by Nesidiocoris tenuis. Int J Mol Sci 17:6–8. https://doi.org/10.3390/ijms17081210
Naselli M, Zappalà L, Gugliuzzo A et al (2017) Olfactory response of the zoophytophagous mirid Nesidiocoris tenuis to tomato and alternative host plants. Arthropod-Plant Interact 11:121–131. https://doi.org/10.1007/s11829-016-9481-5
Pérez-Hedo M, Urbaneja-Bernat P, Jaques JA et al (2015) Defensive plant responses induced by Nesidiocoris tenuis (Hemiptera: Miridae) on tomato plants. J Pest Sci 88:543–554. https://doi.org/10.1007/s10340-014-0640-0
Pérez-Hedo M, Suay R, Alonso M et al (2017) Resilience and robustness of IPM in protected horticulture in the face of potential invasive pests. Crop Prot 97:119–127. https://doi.org/10.1016/j.cropro.2016.11.001
Pérez-Hedo M, Arias-Sanguino ÁM, Urbaneja A (2018a) Induced tomato plant resistance against Tetranychus urticae triggered by the phytophagy of Nesidiocoris tenuis. Front Plant Sci 9:1–8. https://doi.org/10.3389/fpls.2018.01419
Pérez-Hedo M, Rambla JL, Granell A, Urbaneja A (2018b) Biological activity and specificity of Miridae-induced plant volatiles. Biocontrol 63:203–213. https://doi.org/10.1007/s10526-017-9854-4
Pérez-Hedo M, Riahi C, Urbaneja A (2021) Use of zoophytophagous mirid bugs in horticultural crops: current challenges and future perspectives. Pest Manag Sci 77:33–42. https://doi.org/10.1002/ps.6043
Pérez-Hedo M, Bouagga S, Zhang NX et al (2022) Induction of plant defenses: the added value of zoophytophagous predators. J Pest Sci. https://doi.org/10.1007/s10340-022-01506-3
Pérez-Hedo M, Urbaneja A (2016) The zoophytophagous predator Nesidiocoris tenuis: a successful but controversial biocontrol agent in tomato crops. In: Advances in insect control and resistance management, Springer, pp 121–138
Picanço M, Leite G, Guedes R, Silva E (1998) Yield loss in trellised tomato affected by insecticidal sprays and plant spacing. Crop Prot 17:447–452
Polaszek A, Rugman-Jones PF, Stouthamer R et al (2012) Molecular and morphological diagnoses of five species of Trichogramma: biological control agents of Chrysodeixis chalcites (Lepidoptera: Noctuidae) and Tuta absoluta (Lepidoptera: Gelechiidae) in the Canary Islands. Biocontrol 57:21–35. https://doi.org/10.1007/s10526-011-9361-y
Ponti L, Gutierrez AP, de Campos MR et al (2021) Biological invasion risk assessment of Tuta absoluta: mechanistic versus correlative methods. Biol Invasions 23:3809–3829
PubChem PubChem. https://pubchem.ncbi.nlm.nih.gov/. Accessed 16 May 2023
R Core Team (2022) R: a language and environment for statistical computing
Raghava T, Ravikumar P, Hegde R, Kush A (2010) Spatial and temporal volatile organic compound response of select tomato cultivars to herbivory and mechanical injury. Plant Sci 179:520–526. https://doi.org/10.1016/j.plantsci.2010.07.020
Rambla JL, Alfaro C, Medina A et al (2015) Tomato fruit volatile profiles are highly dependent on sample processing and capturing methods. Metabolomics 11:1708–1720. https://doi.org/10.1007/s11306-015-0824-5
Rasulov B, Talts E, Niinemets Ü (2019) A novel approach for real-time monitoring of leaf wounding responses demonstrates unprecedently fast and high emissions of volatiles from cut leaves. Plant Sci 283:256–265. https://doi.org/10.1016/j.plantsci.2019.03.006
Silva DB, Weldegergis BT, Van Loon JJA, Bueno VHP (2017) Qualitative and quantitative differences in herbivore-induced plant volatile blends from tomato plants infested by Either Tuta absoluta or Bemisia tabaci. J Chem Ecol 43:53–65. https://doi.org/10.1007/s10886-016-0807-7
Sylla S, Brévault T, Bal AB et al (2017) Rapid spread of the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae), an invasive pest in Sub-Saharan Africa. Entomol Generalis 36:269–283. https://doi.org/10.1127/entomologia/2017/0453
Thévenot EA, Roux A, Xu Y et al (2015) Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS Statistical Analyse. J Proteome Res 14:3322–3335. https://doi.org/10.1021/acs.jproteome.5b00354
Urbaneja A, González-Cabrera J, Arno J, Gabarra R (2012) Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag Sci 68:1215–1222
Urbaneja A, Coll M, Jaques JA et al (2022) Special issue on recent advances in zoophytophagous arthropods for agroecosystems sustainability. J Pest Sci. https://doi.org/10.1007/s10340-022-01563-8
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20. https://doi.org/10.1007/s10526-011-9395-1
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, New York
Funding
Open access funding provided by Consiglio Nazionale Delle Ricerche (CNR) within the CRUI-CARE Agreement. This research was supported by National Research Council of Italy, Institute for Sustainable Plant Protection (IPSP) through project CNR-IPSP DBA.AD002.356 Lotta Biologica ed Integrata and partially through the project ASTER (Partnership for Research and Innovation in the Mediterranean Area—PRIMA—Section2–Multi-topic 2021—Call 2021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This research complied with all local and national standards for ethical conduct in research. This research did not involve vertebrates or humans; thus, no IRB approvals were needed.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Communicated by Nicolas Desneux.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article has been revised: the authorship has been revised, Pasquale Cascone, Fatemeh Tabebordbar, Gabriele Cencetti, Marco Michelozzi, Parviz Shishehbor, Emilio Guerrieri & Massimo Giorgini.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cascone, P., Tabebordbar, F., Cencetti, . et al. Phytophagy of Nesidiocoris tenuis triggers the response of Trichogramma achaeae to tomato plants infested by Tuta absoluta. J Pest Sci 97, 323–333 (2024). https://doi.org/10.1007/s10340-023-01647-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01647-z