Abstract
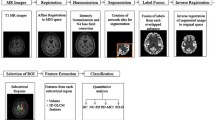
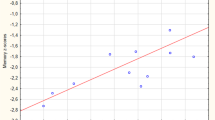
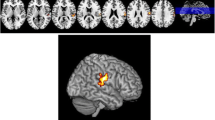
Cognitive impairment in Parkinson’s disease (PD) is associated with changes in the brain anatomical structures. The objective of this study, is to identify the atrophy patterns based on the severity of cognitive decline and evaluate the disease progression. In this study, gray matter alterations are analysed in 135 PD subjects under 3 cognitive domains (91 Cognitively normal PD (NC-PD), 25 PD with Mild Cognitive Impairment (PD-MCI) and 19 PD with Dementia (PD-D)) by comparing them with 58 Healthy Control (HC) subjects. Voxel Based Morphometry (VBM) is used to segment the gray matter regions in magnetic resonance images and analyse the atrophy patterns statistically. Significant patterns of gray matter variations observed in the middle temporal and medial frontal region differentiate between HC and PD subject groups based on the severity of cognitive decline. Abnormalities in gray matter is substantiated through radiomic features extracted from the significant gray matter clusters. Significant radiomic features of the clusters are able to differentiate between the HC and PD-D subjects with an accuracy of 81.82%. Higher atrophy levels identified in PD-D subjects compared to NC-PD and PD-MCI group enables early diagnosis and treatment procedures. The combined and comprehensive analysis of gray matter alterations through VBM and radiomic features gives better assessment of cognitive impairment in PD.









Similar content being viewed by others
References
Aarsland D (2016) Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinsonism Relat Disord 22:144–148
Amoroso N, La Rocca M, Monaco A, Bellotti R, Tangaro S (2018) Complex networks reveal early MRI markers of Parkinson‟s disease. Med Image Anal 48:12–24
Ashburner J, Friston KJ (2000) Voxel-based morphometry-the methods. Neuroimage 11(6):805–821
Beyer MK, Janvin CC, Larsen JP, Aarsland D (2007) A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry 78:254–259
Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA (2010) Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain 133:1747–1754
Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, Shults CW, Stoessl AJ (2003) Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson’s disease. Exp Neurol 184:68–79
Cai D, Zhang C, He X (2010) Unsupervised feature selection for multi-cluster data. In: Proceedings of the 16th ACM SIGKDD international conference on Knowledge discovery and data mining, pp 333–342
Cao X, Wang X, Xue C, Zhang S, Huang Q, Liu W (2020) A radiomics approach to predicting Parkinson’s disease by incorporating whole-brain functional activity and gray matter structure. Front Neurosci 14:751
Cheng Z, Zhang J, He N, Li Y, Wen Y, Xu H, Tang R, Jin Z, Haacke EM, Yan F, Qian D (2019) Radiomic features of the nigrosome-1 region of the substantia nigra: using quantitative susceptibility mapping to assist the diagnosis of idiopathic Parkinson’s disease. Front Aging Neurosci 11:167
Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, Gitelman D, Goldman J, Growdon J, Hurtig HI, Levin B, Litvan I (2010) A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov Disord 25(15):2501–2507
Cigdem O, Demirel H (2018) Performance analysis of different classification algorithms using different feature selection methods on Parkinson’s disease detection. J Neurosci Methods 309:81–90
Davis AA, Racette B (2016) Parkinson disease and cognitive impairment: five new things. Neurol Clin Pract 6(5):452–458
Farokhian F, Beheshti I, Sone D, Matsuda H (2017) Comparing CAT12 and VBM8 for detecting brain morphological abnormalities in temporal lobe epilepsy. Front Neurol 8:428
Grolez G, Viard R, Lopes R, Kuchcinski G, Defebvre L, Devos D, Dujardin K, Moreau C (2020) Functional correlates of cognitive slowing in Parkinson’s disease. Parkinsonism Relat Disord 76:3–9
Gul A, Perperoglou A, Khan Z, Mahmoud O, Miftahuddin M, Adler W, Lausen B (2018) Ensemble of a subset of kNN classifiers. Adv Data Anal Classif 12(4):827–840
Hall JM, Lewis SJ (2019) Neural correlates of cognitive impairment in Parkinson’s disease: a review of structural MRI findings. Int Rev Neurobiol 144:1–28
Halliday GM, McCann H (2010) The progression of pathology in Parkinson’s disease. Ann NY Acad Sci 1184(1):188–195
Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D (2009) Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73(21):1738–1745
Hopes L, Grolez G, Moreau C, Lopes R, Ryckewaert G, Carrière N, Auger F, Laloux C, Petrault M, Devedjian JC, Bordet R (2016) Magnetic resonance imaging features of the nigrostriatal system: biomarkers of Parkinson‟s disease stages? PLoS ONE 11(4):e0147947
Huang H, Zheng S, Yang Z, Wu Y, Li Y, Qiu J, Cheng Y, Lin P, Lin Y, Guan J, Mikulis DJ (2023) Voxel-based morphometry and a deep learning model for the diagnosis of early Alzheimer’s disease based on cerebral gray matter changes. Cereb Cortex 33(3):754–763
Li X, Xing Y, Martin-Bastida A, Piccini P, Auer DP (2018) Patterns of grey matter loss associated with motor subscores in early Parkinson’s disease. NeuroImage Clin 17:498–504
Li Y, Jiang J, Lu J, Jiang J, Zhang H, Zuo C (2019) Radiomics: a novel feature extraction method for brain neuron degeneration disease using 18F-FDG PET imaging and its implementation for Alzheimer‟s disease and mild cognitive impairment. Ther Adv Neurol Disord 12:1756286419838682
Li L, Ji B, Zhao T, Cui X, Chen J, Wang Z (2022) The structural changes of gray matter in Parkinson disease patients with mild cognitive impairments. PLoS ONE 17(7):e0269787
Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, Coffey C, Kieburtz K, Flagg E, Chowdhury S, Poewe W (2011) The Parkinson progression marker initiative (PPMI). Prog Neurobiol 95(4):629–635
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699
Nie K, Gao Y, Mei M, Guo M, Huang Z, Wang L, Zhao J, Zhang Y, Wang L (2019) The clinical characteristics and cognitive features of mild cognitive impairment in Parkinson’s disease and the analysis of relevant factors. J Clin Neurosci 63:142–148
Pang H, Yu Z, Li R, Yang H, Fan G (2020) MRI-based radiomics of basal nuclei in differentiating idiopathic Parkinson’s disease from parkinsonian variants of multiple system atrophy: a susceptibility-weighted imaging study. Front Aging Neurosci 12:587250
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 3(17013):1–21
Provost JS, Hanganu A, Monchi O (2015) Neuroimaging studies of the striatum in cognition Part I: healthy individual. Front Syst Neurosci 9:140
Ramaniharan AK, Manoharan SC, Swaminathan R (2016) Laplace Beltrami eigen value based classification of normal and Alzheimer MR images using parametric and non-parametric classifiers. Expert Syst Appl 59:208–216
Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A (2014) Grey matter changes in cognitively impaired Parkinson’s disease patients. PLoS ONE 9(1):e85595
Salvatore C, Cerasa A, Castiglioni I, Gallivanone F, Augimeri A, Lopez M, Arabia G, Morelli M, Gilardi MC, Quattrone A (2014) Machine learning on brain MRI data for differential diagnosis of Parkinson’s disease and progressive supranuclear palsy. J Neurosci Methods 222:230–237
Sampedro F, Marín-Lahoz J, Martínez-Horta S, Pagonabarraga J, Kulisevsky J (2018) Early gray matter volume loss in MAPT H1H1 de Novo PD patients: a possible association with cognitive decline. Front Neurol 9:394
Siciliano M, De Micco R, Giordano A, Di Nardo F, Russo A, Caiazzo G, De Mase A, Cirillo M, Tedeschi G, Trojano L, Tessitore A (2020) Supplementary motor area functional connectivity in “drug-naïve” Parkinson’s disease patients with fatigue. J Neural Transm 127(8):1133–1142
Simuni T, Caspell-Garcia C, Coffey CS, Weintraub D, Mollenhauer B, Lasch S, Tanner CM, Jennings D, Kieburtz K, Chahine LM, Marek K (2018) Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson’s disease: the PPMI cohort. J Neurol Neurosurg Psychiatry 89(1):78–88
Sivaranjini S, Sujatha CM (2021) Morphological analysis of subcortical structures for assessment of cognitive dysfunction in Parkinson’s disease using multi-atlas based segmentation. Cogn Neurodyn 15(5):835–845
Sun D, Wu X, Xia Y, Wu F, Geng Y, Zhong W, Zhang W, Guo D, Li C (2021) Differentiating Parkinson’s disease motor subtypes: a radiomics analysis based on deep gray nuclear lesion and white matter. Neurosci Lett 760:136083
Tröster AI (2008) Neuropsychological characteristics of dementia with Lewy bodies and Parkinson’s disease with dementia: differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychol Rev 18:103–119
Vallières M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60(14):5471
Wolters AF, Moonen AJ, Lopes R, Leentjens AF, Duits AA, Defebvre L, Delmaire C, Hofman PA, Van Bussel FC, Dujardin K (2020) Grey matter abnormalities are associated only with severe cognitive decline in early stages of Parkinson’s disease. Cortex 123:1–1
Wu T, Hallett M (2013) The cerebellum in Parkinson’s disease. Brain 136(3):696–709
Wu Y, Jiang JH, Chen L, Lu JY, Ge JJ, Liu FT, Yu JT, Lin W, Zuo CT, Wang J (2019) Use of radiomic features and support vector machine to distinguish Parkinson’s disease cases from normal controls. Ann Transl Med 7(23):773
Xia J, Miu J, Ding H, Wang X, Chen H, Wang J, Wu J, Zhao J, Huang H, Tian W (2013) Changes of brain gray matter structure in Parkinson’s disease patients with dementia. Neural Regener Res 8(14):1276–1285
Zeng H, Cheung YM (2011) Feature selection and kernel learning for local learning-based clustering. IEEE Trans Pattern Anal Mach Intell 33(8):1532–1547
Acknowledgements
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI is a public-private partnership, funded by The Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Allergan, Amathus, Avid, Biogen, BioLegend, Bristol-Myers Squibb, Celgene, Denali, GE Healthcare, Genentech, GSK, Golub Capital, Handle Therapeutics, Insitro, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi Genzyme, Servier, Takeda, TEVA, UCB, Verily and Voyager Therapeutics (www.ppmi-info.org/fundingpartners).
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments after approval of the local ethics committees of the sites participating to the Parkinson’s Progression Markers Initiative (PPMI). PPMI is a multicentric longitudinal study. Detailed information is available at http://ppmi-info.org/ppmiclinical-sites. The relevant local institutional review boards approved the PPMI protocol and written informed consent was obtained from all participants prior to inclusion. The approval for the use of the data in the current study was given by the Parkinson’s Progression Markers Initiative. No additional ethics approval was required from the local ethics committee where data was analysed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Editor: Giulia Liberati (Catholic University of Louvain); Reviewers: G Venugopal (Indian Institute of Technology Madras), Sebastiano Vacca (University of Cagliari).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sivaranjini, S., Sujatha, C.M. Analysis of cognitive dysfunction in Parkinson’s disease using voxel based morphometry and radiomics. Cogn Process (2024). https://doi.org/10.1007/s10339-024-01197-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10339-024-01197-x