Abstract
Plumage ornaments of birds, both pigment-based and structural, are considered age or condition-dependent, honestly signalling male quality, sexually selected traits, influencing the variation in breeding performance as well as adaptive sex allocation. In the present study, we examine the effect of age of males on the plumage structural colour in Common Kingfisher, and evaluate the breeding output (laying date, total number of young per breeding season, mean number of young per nest, total number of broods) and sex ratio in relation to two ornamental traits (forehead and rump structural colour). During the three years 2016–2018 in the Danube river system (south-western Slovakia), we collected data from 49 males, 102 broods and 645 nestlings. Our data demonstrate that structural colour is a condition-related and age-sensitive signal, while males with increased weight expressed less saturated blue forehead feathers, and old males displayed more saturated cyan rump feathers than young ones. Moreover, the brood sex ratio varies with male coloration in an age-dependent manner. The plumage colour of young males did not affect the brood sex ratio, whereas old males with increasing intensity of cyan rump had more sons than duller ones. Neither single ornamental trait nor age predicted breeding output of kingfisher males. Nevertheless, our results indicate that the blue structural plumage of kingfisher males may serve as an indicator of age and a certain kind of quality with a consequence on adaptive sex allocation in this species.
Zusammenfassung
Alter und Gefiederfärbung des Männchens bestimmen das Geschlechterverhältnis beim Eisvogel (Alcedo atthis).
Der Gefiederschmuck von Vögeln, sowohl pigmentbasiert als auch strukturell, gilt als alters- oder konditionsabhängig und signalisiert ehrlich die Qualität der Männchen, sexuell selektierte Merkmale, die die Variation der Brutleistung sowie die adaptive Geschlechterverteilung beeinflussen. In der vorliegenden Studie untersuchen wir die Auswirkung des Alters der Männchen auf die Gefiederstrukturfarbe des Eisvogels und bewerten den Bruterfolg (Legedatum, Gesamtzahl der Jungen pro Brutsaison, durchschnittliche Anzahl der Jungen pro Nest, Gesamtzahl der Bruten) und das Geschlechterverhältnis in Bezug auf zwei Ziermerkmale (Stirn- und Bürzeltrukturfarbe). Während der drei Jahre 2016-2018 haben wir im Donauraum (SW Slowakei) Daten von 49 Männchen, 102 Bruten und 645 Nestlingen gesammelt. Unsere Daten zeigen, dass die Strukturfarbe ein konditionsabhängiges und altersempfindliches Signal ist, wobei Männchen mit höherem Gewicht weniger gesättigte blaue Stirnfedern und alte Männchen mehr gesättigte cyanfarbene Bürzelfedern aufweisen als junge. Außerdem variiert das Geschlechterverhältnis bei der Brut mit der Färbung der Männchen in einer altersabhängigen Weise. Die Gefiederfarbe der jungen Männchen hatte keinen Einfluss auf das Geschlechterverhältnis in der Brut. Hingegen hatten alte Männchen mit zunehmender Intensität des cyanfarbenen Bürzels mehr Söhne als mattere Männchen. Weder ein einzelnes Ziermerkmal noch das Alter sagten die Brutleistung von Eisvogel-Männchen voraus. Dennoch deuten unsere Ergebnisse darauf hin, dass das blaue Strukturgefieder der Eisvogelmännchen als Indikator für das Alter und eine bestimmte Art von Qualität dienen kann, was wiederum Auswirkungen auf die adaptive Geschlechtszuweisung bei dieser Art hat.
Similar content being viewed by others
Introduction
Birds are the most colourful group of terrestrial vertebrates (Stoddard and Prum 2011). Their spectacular plumage coloration, often more elaborate in males, is produced by pigments—mostly melanin (providing structural support to feather, Burtt 1981) and carotenoids (essential vitamin A precursors, Underwood 1984), physical interaction of light with feather structures (Prum and Torres 2003) or a combination of those two mechanisms (Hill 2006).
The role of pigment-based coloration as a signal of male quality is well studied in birds (Hill 2006; Mc Graw 2008; Guindre-Parker and Love 2014). On the other side, structure-based coloration has received much less attention (e.g. McGraw et al. 2002; Hill et al. 2005) and caused more controversy, since the self-assembly of the nanostructure reduces the potential for condition-dependent trait expression in structural coloration during feather development (Prum 2006; Prum et al. 2009). However, other features of the barb’s cortex with melanosomes distribution may influence the colour variation produced by the feather (Shawkey et al. 2003). Hence, the honesty of structural signals may be maintained by the physiologically costly investment. The view that structure-based coloration can serve as a honest signal of male quality was supported by results of previous studies, founding more elaborate structure-based coloration in older males, those in better body condition or in males maintaining high-quality territories (Keyser and Hill 1999, 2000; Doucet 2002; Siefferman et al. 2005; Griggio et al. 2010a; Hyun-Young et al. 2016). Evidence also suggests that structural coloration may serve as a reliable indicator of individual quality (White 2020) in male’s interactions (Alonso-Alvarez et al. 2004) as well as in mate choice (Andersson et al. 1998; Johnsen et al. 1998; Keyser and Hill 2000; Pearn et al. 2001; but see Ballentine and Hill 2003; Liu et al. 2007, 2009; Griggio et al. 2010b).
If the structural-based coloration serves as honest signal of male quality, females paired with more ornamented males should experience greater breeding performance benefiting from high-quality territory (Keyser and Hill 2000), high male investment in parental care (Siefferman and Hill 2003) and/or high-quality genotype inherited by their offspring from superior males (Norris 1993). Further, females can increase investment into offspring produced with high-quality males and achieve high reproductive success (Harris and Uller 2009).
Additionally, these females could manipulate the sex ratio of offspring in an attempt to increase lifetime reproductive success (Ellegren et al. 1996). This may be true when one sex is more valuable than other (Fisher 1958; Hamilton 1967; Charnov 1982; Sheldon 1998). In such cases, females in better condition, breeding in high-quality environments or paired with high-quality males or males who are able or willing to invest more in parental care thus should produce high-quality sons who might be more successful in competing for access to females (Trivers and Willard 1973; Clutton-Brock 1984; Svensson and Nilsson 1996; Bowers et al. 2013). Similarly, females mated with more attractive males should preferably produce sons which would inherit attractiveness of their fathers (Burley 1981; Ellegren et al. 1996; Sheldon et al. 1999; Griffith et al. 2003, but see Parker 2013). If inherited traits have a greater effect on the reproductive fitness of male than on female, sons would benefit more than daughters by inheriting attractiveness of their father (West and Sheldon 2002, but see Ewen et al. 2004; Booksmythe et al. 2017).
In this study, we focused on structural plumage coloration in the Common Kingfisher (Alcedo atthis). We used data collected across three breeding seasons to assess the relationship between males’ structure-based coloration, condition, age, breeding output and sex ratio. If plumage coloration is a good predictor of male quality, we expect to find superior males (those in better body condition/older) having more elaborate plumage coloration. Further, if male coloration is a sexually selected trait, we predict higher reproductive success in males with more elaborate plumage. Finally, if inherited coloration will have a greater positive effect on the reproductive fitness of sons than daughters, we expect to find the sex ratio shifted towards sons in broods of males with more elaborate plumage coloration.
Material and methods
Studied species
In the Common Kingfisher, both males and females express a very distinctive cyan feather ornament, which is mainly structural. Cyan feathers of the head and back consist of cyan and blue barbs containing a keratinous spongy nanostructure. Nanostructures create iridescence and are likely to have a structural basis. These feathers have a high reflectance below 500 nm and a low reflectance above 600 nm (Stavenga et al. 2011). Males are known to erect blue feathers on their foreheads during aggressive male–male interactions and to show female brightly coloured rump feathers as a part of the courtship display (Cramp 1985).
The distribution of the Common Kingfisher extends across the whole Palearctic, breeding in most of Europe (Keller et al. 2020). It depends on the open water for feeding and the steep soil banks, in which they dig a breeding tunnel (Cramp 1985; Turčoková et al. 2016). Populations in Central Europe breed from March to September (Čech 2010; Rubáčová et al. 2020). They are mostly socially monogamous, with the occurrence of polygamy and production of extra-pair young (Cramp 1985; Woodall 2001; Čech 2009a; Libois 2018; Cepková et al. 2022a). Females lay up to four clutches per breeding season with two being the most common (Rubáčová et al. 2020), while their consecutive breeding attempts usually overlap (Čech 2010; Rubáčová et al. 2020). High offspring production apparently compensates for the rather low winter survival (Rubáčová et al. 2021). Both sexes dig a breeding tunnel, incubate eggs and care for young (Cramp 1985; Woodall 2001). Nestling sex ratio is equal within the population, while an obvious shift in individual broods remains unexplained (Cepková et al. 2019, 2022b).
Fieldwork
We studied Common Kingfishers from the beginning of April to late September during the breeding seasons 2016–2018 in the Danube river system (south-western Slovakia), particularly between 1868.7 (Bratislava, 48° 06′ 13.5″ N 17° 09′ 31.3″ E) and 1819.0 river km (Gabčíkovo, 47° 52′ 32.1″ N 17° 31′ 18.0″ E). We were looking for nests from the boat. Active nests were inspected weekly using a miniature camera (Probe Maxivideo MV 201).
After hatching, parents were captured using mist-nets installed near their nesting hole, and both members of the breeding pair were ringed and blood sampled. Males were subsequently aged as a second year (hereafter young) and after second year (hereafter old) following Čech (2009b), weighted and their tarsus length was measured. To obtain feathers for later spectral analyses, we cut ten feathers from the forehead and rump of each male. We stored the feathers in separate envelopes in a climate-controlled environment until further processing.
When nestlings were at least 12 days old (range 12–25, mean 16 days, N = 645), we used bent iron wire to gently take them out of the nest chamber. After ringing, we took a small blood sample (25 μl) from each nestling for later sex determination and paternity analyses. Blood samples were subsequently suspended in 96% ethanol and frozen in a plastic tube.
To assess the number of broods per breeding season within a breeding pair, we continuously checked all marked breeding pairs until the end of the breeding season. The fieldwork was completed at the end of September after all the young had fledged.
Body condition
Individual condition was assessed by calculating values for body condition (defined by scaled mass index; SMI). SMI was calculated for each individual using a scaling exponent (estimated by the standardised major axis regression of body mass on linear body measurement, in our study tarsus length), arithmetic mean value of tarsus length and log-transformed body mass (Peig and Green 2009).
Spectral analyses
Ten feathers from both patches (forehead, rump) were used for spectral analyses. We arranged feathers so that they overlapped extensively and took three readings from different parts of each set of feathers. All readings were taken using an Ocean Optics JAZ spectrometer (Ocean Optics Inc., Dunedin, FL, USA) and the micron fibre-optic probe at a 90° angle to the feather surface. We averaged patch-specific spectra for each individual, which resulted in one average reflectance spectrum for each patch and bird. We used the R package “pavo” (Maia et al. 2013) to calculate summary spectral characteristics for each feather patch. In particular, we calculated the hue (spectral location) of the rump and forehead as a wavelength of maximum reflectance λ(Rmax). Chroma (spectral purity) was calculated as a relative contribution of a spectral range to the total brightness. Since patches of blue feathers are also reflected in the UV range, we calculated “blue chroma” (R400–510/R320–700) and “UV chroma” (R320–400/R320–700) (White and Cristol 2014). For both patches, we calculated brightness (spectral intensity) as mean relative reflectance over the entire spectral range (Montgomerie 2006).
Molecular procedures
In total, 645 nestlings from 49 males and 102 nests were sexed (Table 1). DNA was extracted from blood samples by E.Z.N.A.® Tissue DNA Kit. Subsequently, PCR amplification and electrophoresis were performed to identify the sex of nestlings. The volume of PCR mix was 10 µl. Each sample contained: 2 µl DNA, 0.2 µl of primer sex1 and sex2, 5 µl Master Mix (VWR Red Taq DNA Polymerase Master Mix) and 2.6 µl deionized water. We also performed PCR reaction with primers P2 and P8 (Griffiths et al. 1998), but the sex1, sex2 method had a better outcome. Primers and procedures were done according to Wang and Zhang (2009). Electrophoresis was performed 60 min at 100 V on 2% agarose gel containing GoodView. Then, UV light was used to visualize the presence of bands: two bands identifying female and one band identifying male. Sex was determined for all nestlings we sampled. There was also a control sample of one adult male and one adult female for each electrophoresis.
For parentage analyses, the forward primers were fluorescently labelled, and multiplex PCR kit (QIAGEN Multiplex PCR Plus kit) was used to amplify four microsatellite loci (AACC-106, Be2.46, Bb111, CAM17). Fragment analysis was carried out commercially in the Comenius University Science Park (Bratislava, Slovakia) and its results were visualised by software GeneMarker. Alleles of putative parents were compared to those of nestlings to determine whether they were or were not within-pair offspring. Nestlings failed to be considered as within-pair if their alleles mismatched with those of their parent at least in one locus (see Cepková et al. 2022a).
Statistical analyses
We tested data for normality (Shapiro–Wilk W test). Data lacking normal distribution were ln-transformed (brightness forehead, UV chroma rump, laying date). Transformed data approached a normal distribution.
To reduce spectral data recorded from two body regions to a limited number of variables, we performed principal component analyses (PCA) with varimax rotation, based on correlation matrices of standardized plumage characteristics (Table 2). The varimax rotation simplified the loadings of items by removing the middle ground and more specifically identified the factor upon which data load. Principal component analysis reduced the number of colour variables into a more manageable number (from eight variables to two components). In total, two principal components explained 74.2% of variation among colour variables. First principal component (PC1) explained 40.6% of the variation in data and received strong loadings from forehead structural blue coloration variables. An individual with a high positive PC1 score has more blue as well as more UV saturation in forehead feathers (colour appears more intense blue and brighter). Second principal component (PC2) explained 33.6% of the variation and received strong loadings from rump structural blue coloration variables. An individual with a high positive PC2 score for rump colour has feathers with greater blue saturation and with a more left-shifted hue (colour appears more intense cyan).
To determine which parameter best explains variation in phenotypic characteristics of males we used generalised linear models. We set year of the study, male age and scaled mass index (SMI) as predictors and two principal components as a continuous dependent variable.
First egg-laying date was defined as the date when the female laid her first egg. It was measured as Julian date, while in each year we determined the start of the breeding season by the earliest day of the first egg-laying date in population, and assign this day as number 1 (hereafter we will use the laying date as the parameter name). We quantified seasonal reproductive output as the total number of offspring fledged from all broods per breeding season to a specific male (hereafter nestling number), and total number of broods belonging to a specific male per breeding season (hereafter brood number). As these two characteristics were highly correlated (Pearson’s r = 0.976; p < 0.001; n = 49), we used only nestling number/breeding season in the analyses.
To investigate whether the year of study, age and phenotypic characteristics of males predicted variation in their seasonal reproductive output (response variable: laying date, nestling number/breeding season), we used generalized linear models with normal distribution and identity link function (McCullagh and Nelder 1989). Year of study and the age of a male were set as a categorical predictors and two principal components were set as continuous predictors. As each male was used only once in the analyses, we did not set male identity as a random factor. In addition, we performed generalized linear mixed model with normal distribution and identity link function to examine the correlation between the number of young in each brood (nestling number/nest) and male age, year of study and phenotypic traits of the males. Male age and year of study were set as categorical predictors, two principal components of plumage coloration were set as continuous predictors and pull number/nest was set as a response variable. As males have typically more than one brood per breeding season, male identity was set as a random factor.
Sex ratio was defined as the proportion of males to the total number of nestlings in the nest. Brood sex ratio was analysed using generalized linear mixed model with binomial error distribution and logit link function (Krackow and Tkadlec 2001). Sex ratio was set as a response variable. Male’s age and year of study (fixed effects) were set as a categorical predictors and two principal components obtained from colour measurements were set as continuous predictors. All statistical analyses were performed using the statistical program STATISTICA for WINDOWS v. 8 (StatSoft Inc., Tulsa, OK, USA) ans IBM SPSS Statistics v. 23 (Armonk, NY: IBM Corp.).
Results
Kingfisher males had mean 1.91 ± 0.10 SE broods per breeding season (range 1–5 broods, N = 102 broods) with the mean number of nestlings per brood was 6.32 ± 0.94 SE (range 3–8 nestlings, N = 102 broods).
Male ornamentation in relation to age and body condition
Male body condition was negatively correlated with blue structural coloration of the forehead (PC1). Males with a higher PC1 score (greater blue and UV chroma in forehead feather) have a lower value of SMI (Fig. 1). No correlation has been found between the body condition and structural coloration of the rump (PC2).
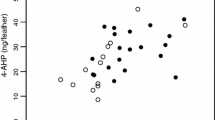
Male age was not correlated with blue structural coloration of the forehead (PC1), but was a significant predictor of blue structural coloration of the rump (PC2). Old males displayed significantly more elaborate rump coloration consisting of more cyan-coloured (more left-shifted hue) and more saturated (with greater blue chroma) and brighter feathers, than young males (Fig. 2a). There was no difference in male coloration between the 3 years the research was conducted (Table 3).
Male age and ornamentation in relation to breeding success and brood sex ratio
Seasonal male reproductive output was not affected by male age, forehead coloration (PC1) or rump coloration (PC2) and did not differ between the three years of research (Table 4).
Males age and rump coloration (PC2) were a good predictors of the brood sex ratio (Table 5), since broods of young males (Fig. 2b) and those with more intense cyan rump feathers (PC2) were more skewed towards sons. In the group of young males, no correlation was found between forehead coloration (PC1), rump coloration (PC2) and brood sex ratio. In the group of old males, those with more intense rump coloration (PC2) had a sex ratio skewed towards sons (Fig. 3). Forehead coloration (PC1) did not significantly affect the brood sex ratio in this group of males (Table 5).
Discussion
In our population of Common Kingfisher, the expression of male structural plumage ornamention was shown to be sensitive to body condition. Males in lower body condition were also those with a more pronounced blue forehead. This runs contrary to previous studies, which found a positive correlation between male structural ornamentation and body condition (Doucet 2002; Siefferman et al. 2005; Griggio et al. 2010a). As body condition interprets variations in energy reserves for a given body size (Peig and Green 2009), a possible explanation is that increased weight, which is traditionally considered better condition in birds (Wendeln and Becker 1999; Robinson et al. 2005; Peig and Green 2009) may indeed be a sign of lower condition for some species. For example, kingfisher males are known to aggressively attack intruders in aerial combat and also perform aerial display to attract females (Cramp 1985; Woodall 2001). It is probably more advantageous for such behaviour to weigh less and be more agile (Székely et al. 2006).
In addition, we found evidence that structural plumage coloration is related to age, with old males displaying more intense cyan rump than the young ones. Those results are in agreement with other studies, showing that young birds display less elaborate plumage compared to old ones (Siefferman et al. 2005; Delhey and Kempeaners 2006; Bitton and Dawson 2008). Such an age-related differences in expression of ornamentation may be due to a within individual increase in ornamentation, correlation between intensity of ornamentation and condition, or correlation between signalling and survival (Jennions et al. 2001; Proulx et al. 2002; Lindström et al. 2009; Grunst et al. 2014; Moore et al. 2015). More specifically, age-related differences in intensity of ornamentation can be caused by differential survival of more ornamented individuals, meaning that often the more ornamented, presumably high-quality individuals show higher apparent survival than less ornamented and low-quality ones (Jenions et al. 2001). More ornamented individuals are thus over-represented in older age classes (Forslund and Pärt 1995). Since body condition does not seem to correlate with rump coloration, and we do not have enough data on coloration and survival analyses, nor data to test individual changes in ornamentation, we can only speculate how this works in kingfishers. Either way, a female kingfisher seems to be able to assess the age of her potential mate using structural colour of his rump as an indicator.
Surprisingly, the breeding output of kingfisher males was not affected by their age with older and younger males did not differ in laying date, number of young per breeding season or number of young per nest. The effect of the male’s age on breeding output can be obscured by the lower survival of this species, leading to a rather short lifespan (Rubáčová et al. 2021). Although it is reported in the literature that kingfishers can live up to 15 years (Woodal 2001), the long-term ringing schemes in Slovakia and Czechia show that only very few males older than four years are represented in the population (Rubáčová unpubl. data, Čech pers. com.). With such a short lifespan, the differences in amount of parental experience between males can be expected to be quite small.
Furthermore, the breeding output of kingfisher males was not affected by their structure-based ornamentation. A possible explanation is that in kingfisher males parental performance is not related to their structure-based ornamentation, similarly as was found in the Bluethroat (Luscinia svecica, Smiseth et al. 2001). However, our results do not mean that females do not have any apparent advantage from mating with more ornamented males. Furthermore, it is necessary to also mention the importance of female and her parental abilities. Although there is no evidence of condition-dependent mate preferences in kingfishers as it can be in other birds (Cotton et al. 2006; Holveck et al. 2011), we do know that investment into parental care and subsequently breeding output in kingfishers is affected by the combination of age and actual condition of both partners (Cepková et al. 2022b), which could obscure the effect of male parental performance on breeding output.
Interestingly, males with more elaborate rump coloration had more sons than daughters, as was also found in other studies (Sheldon et al. 1999; Griffith et al. 2003; Delhey et al. 2007). This is consistent with the sex-allocation theory according to which more attractive males produce more valuable sex, inheriting the attractiveness of their father. The attractiveness is more beneficial for sons as they usually have a higher variance in breeding performance than females (Ellegren et al. 1996; West and Sheldon 2002, but see Ewen et al. 2004; Booksmythe et al. 2017). This may be due to extra-pair mating, polygamy or higher survival rates (Johnsen et al. 1998; Örnborg et al. 2001; Griffith et al. 2003; Barenger et al. 2009), which also applies to the Common Kingfisher (Rubáčová et al.2021; Cepková et al. 2022a).
Surprisingly, in our population, the relationship between brood sex ratio and male coloration was found only in old males. Young males produced more sons than old males, regardless of the rump coloration. Age-related adjustment of the brood sex ratio was documented also in other studies, but explanation remains unclear. Specifically in Blue Tit (Cyanistes caeruleus), a positive effect of female but not male age on brood sex ratio was found only in some years, while not in others (Griffith et al. 2003). In another study, it was the age of the male that affected the sex ratio of the offspring. Young males with more elaborate plumage had more sons, while old brightly ornamented males had more daughters (Delhey et al. 2007).
In conclusion, our results demonstrate that plumage structural coloration of kingfisher males is condition related and age dependent. We also show that the brood sex ratio varies with the age and structural coloration of males. Our work also opens new directions for future studies, specifically in the field of relationship between plumage structural colour of kingfisher males and survival, mate choice, paternal care or habitat quality.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Alonso-Alvarez C, Doutrelant C, Sorci G (2004) Ultraviolet reflectance affects male–male interactions in the blue tit (Parus caeruleus ultramarinus). Behav Ecol 15:805–809. https://doi.org/10.1093/beheco/arh083
Andersson S, Ornborg J, Andersson M (1998) Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc R Soc Lond B 265:445–450. https://doi.org/10.1098/rspb.1998.0315
Ballentine B, Hill GE (2003) Female mate choice in relation to structural plumage coloration in Blue Grosbeaks. Condor 105(3):593–598. https://doi.org/10.1093/condor/105.3.593
Barenger SL, Johnson LS, Masters BS (2009) Sexual selection in a socially monogamous bird: male color predicts paternity success in the Mountain Bluebird, Sialia currucoides. Behav Ecol Sociobiol 63:403–411. https://doi.org/10.1007/s00265-008-0674-5
Bitton PP, Dawson RD (2008) Age-related differences in plumage characteristics of male tree swallows Tachycineta bicolor: hue and brightness signal different aspects of individual quality. J Avian Biol 39:446–452. https://doi.org/10.1111/j.0908-8857.2008.04283.x
Booksmythe I, Mautz B, Davis J, Nakagawa S, Jennions MD (2017) Facultative adjustment of the offspring sex ratio and male attractiveness: a systematic review and meta-analysis. Biol Rev 92:108–134. https://doi.org/10.1111/brv.12220
Bowers EK, Munclinger P, Bures S, Kucerova L, Nadvornik P, Krist M (2013) Cross-fostering eggs reveals that female collared flycatchers adjust clutch sex ratios according to parental ability to invest in offspring. Mol Ecol 22:215–228. https://doi.org/10.1111/mec.12106
Burley N (1981) Sex-ratio manipulation and selection for attractiveness. Science 211:721–722. https://doi.org/10.1126/science.211.4483.721
Burtt EH Jr (1981) The adaptiveness of animal colors. Bioscience 31:723–729. https://doi.org/10.2307/1308778
Čech P (2010) Length of the breeding season of the Eurasian Kingfisher (Alcedo atthis) in the Czech Republic. Sylvia 46:53–61
Čech P (2009a) Paper on knowledge about nesting biology of Kingfisher (Alcedo atthis). In: Čech P (ed) Ledňáček říční (Alcedo atthhis), jeho ochrana a výzkum. Sborník referátů z II. Mezinárodního semináře, Vlašim, pp 118–125
Čech P (2009b) Paper on age and sex determination of Kingfisher (Alcedo atthis). In: Čech P (ed) Ledňáček říční (Alcedo atthhis), jeho ochrana a výzkum. Sborník referátů z II. Mezinárodního semináře, Vlašim, pp 118–125 (in Czech, English abstract)
Cepková M, Balážová M, Melišková M, Rubáčová-Turčoková L (2019) No seasonal variation of the sex ratio in the Common Kingfisher Alcedo atthis broods. Acta Ornithol 54:149–155. https://doi.org/10.3161/00016454AO2019.54.2.002
Cepková M, Melišková M, Rubáčová L (2022a) Low extra-pair paternity and polygamy in the common Kingfisher Alcedo atthis. Ardeola 70:41–58. https://doi.org/10.13157/arla.70.1.2023.ra2
Cepková M, Balážová M, Melišková M, Rubáčová L (2022b) Influence of age and body condition on breeding performance in Common Kingfisher Alcedo atthis. J Ornithol 163:251–261
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Clutton-Brock TH, Albon SD, Guinness FE (1984) Maternal dominance, breeding success and birth sex ratios in red deer. Nature 308:358–360. https://doi.org/10.1038/308358a0
Cotton S, Small J, Pomiankowski A (2006) Sexual selection and condition-dependent mate preferences. Curr Biol 16:R755–R765. https://doi.org/10.1016/j.cub.2006.08.022
Cramp S (1985) The Birds of the Western Palearctic, vol IV: Terns to Woodpeckers. Oxford University Press, Oxford
Delhey K, Kempenaers B (2006) Age differences in blue tit plumage colour: within-individual changes or colour-biased survival? J Avian Biol 37:339–348. https://doi.org/10.1111/j.2006.0908-8857.03655.x
Delhey K, Peters A, Johnsen A, Kempenaers B (2007) Brood sex ratio and male UV ornamentation in blue tits (Cyanistes caeruleus): correlational evidence and an experimental test. Behav Ecol Sociobiol 61:853–862. https://doi.org/10.1007/s00265-006-0314-x
Doucet S (2002) Structural plumage coloration, male body size, and condition in the blue-black grassquit. Condor 104:30–38. https://doi.org/10.1093/condor/104.1.30
Ellegren H, Gustafsson L, Sheldon BC (1996) Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. PNAS 93:11723–11728. https://doi.org/10.1073/pnas.93.21.11723
Ewen JG, Cassey P, Møller AP (2004) Facultative primary sex ratio variation: a lack of evidence in birds? Proc R Soc Lond B 271:1277–1282. https://doi.org/10.1098/rspb.2004.2735
Fisher RA (1958) The Genetical Theory of Natural Selection. Clarendon, Oxford
Forslund P, Pärt T (1995) Age and reproduction in birds-hypotheses and tests. Trends Ecol Evol 10:374378. https://doi.org/10.1016/S0169-5347(00)89141-7
Griffith SC, Örnborg J, Russell AF, Andersson S, Sheldon BC (2003) Correlations between ultraviolet coloration, overwinter survival and offspring sex ratio in the blue tit. J Evol Biol 16:1045–1054. https://doi.org/10.1046/j.1420-9101.2003.00550.x
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075. https://doi.org/10.1046/j.1365-294x.1998.00389.x
Griggio M, Zanollo V, Hoi H (2010a) UV plumage color is an honest signal of quality in male budgerigars. Ecol Res 25:77–82. https://doi.org/10.1007/s11284-009-0632-3
Griggio M, Hoi H, Pilastro A (2010b) Plumage maintenance affects ultraviolet colour and female preference in the budgerigar. Behav Process 84:739–744. https://doi.org/10.1016/j.beproc.2010.05.003
Grunst AS, Rotenberry JT, Grunst ML (2014) Age-dependent relationships between multiple sexual pigments and condition in males and females. Behav Ecol 25:276–287. https://doi.org/10.1093/beheco/art124
Guindre-Parker S, Love OP (2014) Revisiting the condition dependence of melanin-based plumage. J Avian Biol 45:29–33. https://doi.org/10.1111/j.1600-048X.2013.00190.x
Hamilton WD (1967) Extraordinary sex ratios. Science 156:477–488. https://doi.org/10.1126/science.156.3774.477
Harris WE, Uller T (2009) Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Philos Trans R Soc B Biol Sci 364:1039–1048. https://doi.org/10.1098/rstb.2008.0299
Hill GE (2006) Environmental regulation of ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration vol. I: mechanisms and measurements. Harvard University Press, Cambridge, pp 507–560
Hill GE, Doucet SM, Buchholz R (2005) The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim Behav 69:387–394. https://doi.org/10.1016/j.anbehav.2004.03.013
Holveck MJ, Riebel K (2010) Low-quality females prefer low-quality males when choosing a mate. Proc R Soc B Biol Sci 277:153–160. https://doi.org/10.1098/rspb.2009.1222
Hyun-Young N, Lee S, Lee J, Choi Ch, Choe JC (2016) Multiple structural colors of the plumage reflect age, sex, and territory ownership in the Eurasian Magpie Pica pica. Acta Ornithol 51:83–92. https://doi.org/10.3161/00016454AO2016.51.1.007
Jennions MD, Møller AP, Petrie M (2001) Sexually selected traits and adult survival: a meta-analysis. Q Rev Biol 76:3–36. https://doi.org/10.1086/393743
Johnsen A, Andersson T, Ornborg F, Lifjeld J (1998) Ultraviolet plumage coloration affects social mate choice and sperm competition in bluethroats (Aves: Luscinia s. svecica): a field experiment. Proc R Soc Lond B 265:1313–1318. https://doi.org/10.1098/rspb.1998.0435
Keller V, Herrando S, Voríšek P, Franch M, Kipson M, Milanesi P, Martí D, Anton M, Klvaňová A, Kalyakin MV, Bauer HG (2020) European breeding bird atlas 2: distribution, abundance and change. Lynx Edition, Barcelona
Keyser A, Hill G (1999) Condition-dependent variation in the blue ultraviolet coloration of a structurally-based plumage ornament. Proc R Soc Lond B 265:771–777. https://doi.org/10.1098/rspb.1999.0704
Keyser A, Hill G (2000) Structurally-based plumage color is an honest indicator of quality in male blue grosbeaks. Behav Ecol 11:202–209. https://doi.org/10.1093/beheco/11.2.202
Krackow S, Tkadlec E (2001) Analysis of brood sex ratios: implications of offspring clustering. Behav Ecol Sociobiol 50:293–301. https://doi.org/10.1007/s002650100366
Libois R (2018) Plumes d’azur. Histoire naturelle du martin-pêcheur d’Europe. Presses Universitaires de Liége, Gembloux
Lindström J, Pike T, Blount J, Metcalfe N (2009) Optimization of resource allocation can explain the temporal dynamics and honesty of sexual signals. Am Nat 174:515–525. https://doi.org/10.1086/606008
Liu M, Siefferman LM, Hill GE (2007) An experimental test of female choice relative to male structural coloration in eastern bluebirds. Behav Ecol Sociobiol 61:623–630. https://doi.org/10.1007/s00265-006-0292-z
Liu M, Siefferman L, JrH M, Steffen JE, Hill GE (2009) A field test of female mate preference for male plumage coloration in eastern bluebirds. Anim Behav 78:879–885. https://doi.org/10.1016/j.anbehav.2009.07.012
Maia R, Eliason CM, Bitton PP, Doucet SM, Shawkey MD (2013) pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol Evol 4:906–913. https://doi.org/10.1111/2041-210X.12069
McCullagh P, Nelder J (1989) Generalized linear models, 2nd edn. Chapman and Hall, Boca Raton
McGraw KJ (2008) An update on the honesty of melanin-based color signals in birds. Pigment Cell Melanoma Res 21:133–138. https://doi.org/10.1111/j.1755-148X.2008.00454.x
McGraw KJ, Mackillop EA, Dale J, Hauber ME (2002) Different colors reveal different information: how nutritional stress affects the expression of melanin and structurally based ornamental plumage. J Exp Biol 205:3747–3755. https://doi.org/10.1242/jeb.205.23.3747
Montgomerie R (2006) Analyzing colors. In: Hill GE, McGraw KJ (eds) Bird coloration: mechanisms and measurements, vol 2. Harvard University Press, Massachusetts, pp 90–147
Moore FR, Cīrule D, Kivleniece I, Vrublevska J, Rantala MJ, Sild E, Sepp T, Hõrak P, Krama T, Krams I (2015) Investment in a sexual signal results in reduced survival under extreme conditions in the male great tit (Parus major). Behav Ecol Sociobiol 69:151–158. https://doi.org/10.1007/s00265-014-1828-2
Norris K (1993) Heritable variation in a plumage indicator of viability in male great tits Parus major. Nature 362(6420):537–539. https://doi.org/10.1038/362537a0
Örnborg J, Andersson S, Johnsen A, Lifjeld J, Amundsen T (2001) Male characteristics and fertilisation success in bluethroats. Behaviour 138:1371–1390. https://doi.org/10.1163/156853901317367645
Parker TH (2013) What do we really know about the signaling role of plumage colour in blue tits? A case study of impediments to progress in evolutionary biology. Biol Rev 88:511–536. https://doi.org/10.1111/brv.12013
Pearn SM, Bennett AT, Cuthill IC (2001) Ultraviolet vision, fluorescence and mate choice in a parrot, the budgerigar Melopsittacus undulatus. Proc Biol Sci 268:2273–2279. https://doi.org/10.1098/rspb.2001.1813
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891. https://doi.org/10.1111/j.1600-0706.2009.17643.x
Proulx SR, Day T, Rowe L (2002) Older males signal more reliably. Proc R Soc Lond B 269:2291–2299. https://doi.org/10.1098/rspb.2002.2129
Prum RO (2006) Anatomy, physics and evolution of avian structural colours. In: Hill GE, McGraw KJ (eds) Bird coloration: mechanisms and measurements, vol 2. Harvard University Press, Cambridge
Prum RO, Torres RH (2003) A Fourier tool for the analysis of coherent light scattering by bio-optical nanostructures. Integr Comp Biol 43:591–602. https://doi.org/10.1093/icb/43.4.591
Prum RO, Dufresne ER, Quinn T, Waters K (2009) Development of colour-producing keratin nanostructures in avian feather barbs. J R Soc Interface 6:S253–S265. https://doi.org/10.1098/rsif.2008.0466.focus
Robinson S, Chiaradia A, Hindell MA (2005) The effect of body condition on the timing and success of breeding in Little Penguins Eudyptula minor. Ibis 147:483–489. https://doi.org/10.1111/j.1474-919x.2005.00431.x
Rubáčová L, Čech P, Melišková M, Balážová M (2020) The length of breeding season in two populations of the Common Kingfisher (Alcedo atthis). Sylvia 56:39–48
Rubáčová L, Čech P, Melišková M, Čech M, Procházka P (2021) The effect of age, sex and winter severity on return rates and apparent survival in the Common Kingfisher Alcedo atthis. Ardea 109:15–25. https://doi.org/10.5253/arde.v109i1.a2
Shawkey MD, Estes AM, Siefferman LM, Hill GE (2003) Nanostructure predicts intraspecific variation in structural plumage colour. Proc R Soc Lond B 270:1455–1460. https://doi.org/10.1098/rspb.2003.2390
Sheldon BC (1998) Recent studies of avian sex ratios. Heredity 80:397–402. https://doi.org/10.1046/j.1365-2540.1998.00374.x
Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J (1999) Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877. https://doi.org/10.1038/47239
Siefferman LM, Hill GE (2003) Structural and melanin plumage coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav Ecol 14:855–861. https://doi.org/10.1093/beheco/arg063
Siefferman LM, Hill GE (2005) Blue structural coloration of male eastern bluebirds Sialia sialis predicts incubation provisioning to females. J Avian Biol 36:488–493. https://doi.org/10.1111/j.0908-8857.2005.03659.x
Siefferman L, Hill GE, Dobson S (2005) Ornamental plumage coloration and condition are dependent on age in eastern bluebirds Sialia sialis. J Avian Biol 36:428–435. https://doi.org/10.1111/j.0908-8857.2005.03401.x
Smiseth P, Örnborg J, Andersson S, Amundsen T (2001) Is male plumage reflectance correlated with paternal care in bluethroats? Behav Ecol 12:164–170. https://doi.org/10.1093/beheco/12.2.164
Stavenga DG, Tinbergen J, Leertouwer HL, Wilts BD (2011) Kingfisher feathers coloration by pigments, spongy nanostructures and thin films. J Exp Biol 214:3960–3967. https://doi.org/10.1242/jeb.062620
Stoddard MC, Prum RO (2011) How colorful are birds? Evolution of the avian plumage color gamut. Behav Ecol 22:1042–1052. https://doi.org/10.1093/beheco/arr088
Svensson E, Nilsson JA (1996) Mate quality affects offspring sex ratio in blue tits. Proc R Soc Lond B 263:357–361. https://www.jstor.org/stable/50621
Székely T, Thomas GH, Cuthill IC (2006) Sexual conflict, ecology, and breeding systems in shorebirds. Bioscience 56:801–808. https://doi.org/10.1641/0006-3568(2006)56[801:SCEABS]2.0.CO;2
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92. https://doi.org/10.1126/science.179.4068.90
Turčoková L, Melišková M, Balážová M (2016) Nest site location and breeding success of Common kingfisher (Alcedo atthis) in the Danube river system. Folia Oecol 43:74–82
Underwood BA (1984) Vitamin A in animal and human nutrition. In: Sporn MB, Roberts AB, Goodman DS (eds) The retinoids. Academic Press, New York, pp 281–392
Wang N, Zhang ZW (2009) The novel primers for sex identification in the brown eared-pheasant and their application to other species. Mol Ecol Resour 9:186–188. https://doi.org/10.1111/j.1755-0998.2008.02177.x
Wendeln H, Becker PH (1999) Effects of parental quality and effort on the reproduction of common terns. J Anim Ecol 68:205–214. https://doi.org/10.1046/j.1365-2656.1999.00276.x
West SA, Sheldon BC (2002) Constraints in the evolution of sex ratio adjustment. Science 295:1685–1688. https://doi.org/10.1126/science.1069043
White TE (2020) Structural colours reflect individual quality: a meta-analysis. Biol Lett 16:20200001. https://doi.org/10.1098/rsbl.2020.0001
White AE, Cristol DA (2014) Plumage coloration in belted kingfishers (Megaceryle alcyon) at a mercury-contaminated river. Waterbirds 37(2):144-152. https://doi.org/10.1675/063.037.0203
Woodall PF (2001) Family Alcedinidae (Kingfishers). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world. Vol. 6: mousebirds to hornbills. Lynx Edicions, Barcelona
Acknowledgements
We would like to thank Pavel Čech for advices with the fieldwork and Peter Mikulíček for supervising techniques in the molecular laboratory.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This research was supported by grant LIFE12 NAT/SK/001137, UK/138/2019, UK/162/2020.
Study methods comply with the current laws of the country in which they were performed. Permissions to carry out the study were granted by the relevant national authorities of the Ministry of the Environment of the Slovak Republic.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [MC, BM], [MM, LR] and [MB, LR]. The first draft of the manuscript was written by [LR] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lucia, R., Mária, M., Monika, C. et al. Male’s age and plumage coloration predicts brood sex ratio in the Common Kingfisher (Alcedo atthis). J Ornithol 165, 439–448 (2024). https://doi.org/10.1007/s10336-023-02107-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02107-2