Abstract
Objective
The goal of this work is to study the changes in white matter integrity in R6/2, a well-established animal model of Huntington’s disease (HD) that are captured by ex vivo diffusion imaging (DTI) using a high field MRI (17.6 T).
Materials and methods
DTI and continuous time random walk (CTRW) models were used to fit changes in the diffusion-weighted signal intensity in the corpus callosum of controls and in R6/2 mice.
Results
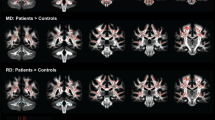
A significant 13% decrease in fractional anisotropy, a 7% increase in axial diffusion, and a 33% increase in radial diffusion were observed between R6/2 and control mice. No change was observed in the CTRW beta parameter, but a significant decrease in the alpha parameter (− 21%) was measured. Histological analysis of the corpus callosum showed a decrease in axonal organization, myelin alterations, and astrogliosis. Electron microscopy studies demonstrated ultrastructural changes in degenerating axons, such as an increase in tortuosity in the R6/2 mice.
Conclusions
DTI and CTRW diffusion models display quantitative changes associated with the microstructural alterations observed in the corpus callosum of the R6/2 mice. The observed increase in the diffusivity and decrease in the alpha CTRW parameter providing support for the use of these diffusion models for non-invasive detection of white matter alterations in HD.




Similar content being viewed by others
References
Harper PS (1992) The epidemiology of Huntington’s disease. Hum Genet 89(4):365–376
Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N (2012) The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov Disord 27(9):1083–1091
Walker FO (2007) Huntington’s disease. Lancet 369(9557):218–228
Han I, You Y, Kordower JH, Brady ST, Morfini GA (2010) Differential vulnerability of neurons in Huntington’s disease: the role of cell type-specific features. J Neurochem 113(5):1073–1091
Perez-Navarro E, Canals JM, Gines S, Alberch J (2006) Cellular and molecular mechanisms involved in the selective vulnerability of striatal projection neurons in Huntington’s disease. Histol Histopathol 21(11):1217–1232
Pouladi MA, Morton AJ, Hayden MR (2013) Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci 14(10):708–721
Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87(3):493–506
Menalled L, El-Khodor BF, Patry M, Suarez-Farinas M, Orenstein SJ, Zahasky B, Leahy C, Wheeler V, Yang XW, MacDonald M, Morton AJ, Bates G, Leeds J, Park L, Howland D, Signer E, Tobin A, Brunner D (2009) Systematic behavioral evaluation of Huntington’s disease transgenic and knock-in mouse models. Neurobiol Dis 35(3):319–336
Ramaswamy S, McBride JL, Kordower JH (2007) Animal models of Huntington’s disease. ILAR J 48(4):356–373
Gatto RG, Chu Y, Ye AQ, Price SD, Tavassoli E, Buenaventura A, Brady ST, Magin RL, Kordower JH, Morfini GA (2015) Analysis of YFP(J16)-R6/2 reporter mice and postmortem brains reveals early pathology and increased vulnerability of callosal axons in Huntington’s disease. Hum Mol Genet 24(18):5285–5298
Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, Fischl B, Pappu V, Onorato C, Cha JH, Salat DH, Hersch SM (2010) Altered white matter microstructure in the corpus callosum in Huntington’s disease: implications for cortical “disconnection”. NeuroImage 49(4):2995–3004
Poudel GR, Stout JC, Dominguez DJ, Churchyard A, Chua P, Egan GF, Georgiou-Karistianis N (2015) Longitudinal change in white matter microstructure in Huntington’s disease: the IMAGE-HD study. Neurobiol Dis 74:406–412
Poudel GR, Stout JC, Dominguez DJ, Salmon L, Churchyard A, Chua P, Georgiou-Karistianis N, Egan GF (2014) White matter connectivity reflects clinical and cognitive status in Huntington’s disease. Neurobiol Dis 65:180–187
Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH (2006) Diffusion tensor imaging in presymptomatic and early Huntington’s disease: selective white matter pathology and its relationship to clinical measures. Mov Disord 21(9):1317–1325
Le Bihan D (2013) Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology 268(2):318–322
Tabesh A, Jensen JH, Ardekani BA, Helpern JA (2011) Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med 65(3):823–836
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61(4):1000–1016
Ingo C, Magin RL, Colon-Perez L, Triplett W, Mareci TH (2014) On random walks and entropy in diffusion-weighted magnetic resonance imaging studies of neural tissue. Magn Reson Med 71(2):617–627
Yu Q, Reutens D, O’Brien K, Vegh V (2016) Tissue microstructure features derived from anomalous diffusion measurements in magnetic resonance imaging. Hum Brain Mapp 38(2):1068–1081
Karaman MM, Sui Y, Wang H, Magin RL, Li Y, Zhou XJ (2016) Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn Reson Med 76(4):1149–1157
Xu B, Su L, Wang Z, Fan Y, Gong G, Zhu W, Gao P, Gao JH (2017) Anomalous diffusion in cerebral glioma assessed using a fractional motion model. Magn Reson Med 78(5):1944–1949
Karaman MM, Wang H, Sui Y, Engelhard HH, Li Y, Zhou XJ (2016) A fractional motion diffusion model for grading pediatric brain tumors. NeuroImage Clinical 12:707–714
Gatto RG, Li W, Magin RL (2018) Diffusion tensor imaging identifies presymptomatic axonal degeneration in the spinal cord of ALS mice. Brain Res 1679:7
Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4(3):316–329
Gatto RG (2018) Diffusion tensor imaging as a tool to detect presymptomatic axonal degeneration in a preclinical spinal cord model of amyotrophic lateral sclerosis. Neural Regen Res 13(3):425–426
Metzler R, Klafter J (2000) The random walk’s guide to anomalous diffusion: a fractional dynamics approach. Phys Rep 339:1–77
Magin RL, Ingo C, Colon-Perez L, Triplett W, Mareci TH (2013) Characterization of anomalous diffusion in porous biological tissues using fractional order derivatives and entropy. Microporous Mesoporous Mater 178:39–43
Klages R, Radons G, Sokolov IM (eds) (2012) Anomalous transport: foundations and applications. Wiley, Weinheim
Magin RL, Abdullah O, Baleanu D, Zhou XJ (2008) Anomalous diffusion expressed through fractional order differential operators in the Bloch–Torrey equation. J Magnetic Resonance 190(2):255–270
Gorenflo RMF, Moretti D, Pagnini G, Paradisi P (2002) Fractional diffusion: probability distributions and random walk models. Phys A 305(1–2):106–112
Wen CSH, Zhanga X, Korosak D (2010) Anomalous diffusion modeling by fractal and fractional derivatives. Comput Math Appl 59(5):1754–1758
Lenzi EK, Ribeiro HV, Tateishi AA, Zola RS, Evangelista LR (2016) Anomalous diffusion and transport in heterogeneous systems separated by a membrane. Proc Math Phys Eng Sci 472(2195):20160502
Klafter J, Lim SC, Metzler R (eds) (2011) Fractional dynamics: recent advances. World Scientific, Sinapore
Mainardi F (2000) Fractional calculus and waves in linear viscoelasticity: an introduction to mathematical models. Imperial College Press, London
Ingo C, Magin RL, Parrish TB (2014) New insights into the fractional order diffusion equation using entropy and kurtosis. Entropy 16(11):5838–5852
Ingo C, Barrick TR, Webb AG, Ronen I (2017) Accurate Padé global approximations for the Mittag–Leffler function, its inverse, and its partial derivatives to efficiently compute convergent power series. Int J Appl Comput Mat 3:347–362
Mount SL, Schwarz JE, Taatjes DJ (1997) Prolonged storage of fixative for electron microscopy: effects on tissue preservation for diagnostic specimens. Ultrastruct Pathol 21(2):195–200
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A–138A
Vranceanu F, Perkins GA, Terada M, Chidavaenzi RL, Ellisman MH, Lysakowski A (2012) Striated organelle, a cytoskeletal structure positioned to modulate hair-cell transduction. Proc Natl Acad Sci USA 109(12):4473–4478
Shaffer JJ, Ghayoor A, Long JD, Kim RE, Lourens S, O’Donnell LJ, Westin CF, Rathi Y, Magnotta V, Paulsen JS, Johnson HJ (2017) Longitudinal diffusion changes in prodromal and early HD: evidence of white-matter tract deterioration. Hum Brain Mapp 38(3):1460–1477
Phillips O, Squitieri F, Sanchez-Castaneda C, Elifani F, Caltagirone C, Sabatini U, Di Paola M (2014) Deep white matter in Huntington’s disease. PLoS One 9(10):e109676
Phillips OR, Joshi SH, Squitieri F, Sanchez-Castaneda C, Narr K, Shattuck DW, Caltagirone C, Sabatini U, DiPaola M (2016) Major superficial white matter abnormalities in Huntington’s disease. Front Neurosci 10:197
Symms M, Jager HR, Schmierer K, Yousry TA (2004) A review of structural magnetic resonance neuroimaging. J Neurol Neurosurg Psychiat 75(9):1235–1244
Höfling F, Franosch T (2013) Anomalous transport in the crowded world of biological cells. Rep Prog Phys 76:046602
Schumer R, Meerschaert MM, Baeumer B (2009) Fractional advection–dispersion equations for modeling transport at the Earth surface. J Geophys Res 114:F00A07
Morton AJ, Glynn D, Leavens W, Zheng Z, Faull RL, Skepper JN, Wight JM (2009) Paradoxical delay in the onset of disease caused by super-long CAG repeat expansions in R6/2 mice. Neurobiol Dis 33(3):331–341
Li H, Li SH, Yu ZX, Shelbourne P, Li XJ (2001) Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Neurosci 21(21):8473–8481
Liu W, Yang J, Burgunder J, Cheng B, Shang H (2016) Diffusion imaging studies of Huntington’s disease: a meta-analysis. Park Relat Disord 32:94–101
Matsui JT, Vaidya JG, Johnson HJ, Magnotta VA, Long JD, Mills JA, Lowe MJ, Sakaie KE, Rao SM, Smith MM, Paulsen JS (2014) Diffusion weighted imaging of prefrontal cortex in prodromal Huntington’s disease. Hum Brain Mapp 35(4):1562–1573
Van Camp N, Blockx I, Camon L, de Vera N, Verhoye M, Veraart J, Van Hecke W, Martinez E, Soria G, Sijbers J, Planas AM, Van der Linden A (2012) A complementary diffusion tensor imaging (DTI)-histological study in a model of Huntington’s disease. Neurobiol Aging 33(5):945–959
Phillips O, Sanchez-Castaneda C, Elifani F, Maglione V, Di Pardo A, Caltagirone C, Squitieri F, Sabatini U, Di Paola M (2013) Tractography of the corpus callosum in Huntington’s disease. PLoS One 8(9):e73280
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45(2):265–269
Weaver KE, Richards TL, Liang O, Laurino MY, Samii A, Aylward EH (2009) Longitudinal diffusion tensor imaging in Huntington’s Disease. Exp Neurol 216(2):525–529
Porrero C, Rubio-Garrido P, Avendano C, Clasca F (2010) Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain Res 1345:59–72
Chan CS, Surmeier DJ (2014) Astrocytes go awry in Huntington’s disease. Nat Neurosci 17(5):641–642
Huang B, Wei W, Wang G, Gaertig MA, Feng Y, Wang W, Li XJ, Li S (2015) Mutant huntingtin downregulates myelin regulatory factor-mediated myelin gene expression and affects mature oligodendrocytes. Neuron 85(6):1212–1226
Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S (2007) Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem Res 32(10):1655–1664
Crawford HE, Hobbs NZ, Keogh R, Langbehn DR, Frost C, Johnson H, Landwehrmeyer B, Reilmann R, Craufurd D, Stout JC, Durr A, Leavitt BR, Roos RA, Tabrizi SJ, Scahill RI (2013) Corpus callosal atrophy in premanifest and early Huntington’s disease. J Huntingt Dis 2(4):517–526
Gomez-Tortosa E, MacDonald ME, Friend JC, Taylor SA, Weiler LJ, Cupples LA, Srinidhi J, Gusella JF, Bird ED, Vonsattel JP, Myers RH (2001) Quantitative neuropathological changes in presymptomatic Huntington’s disease. Ann Neurol 49(1):29–34
Chilla GS, Tan CH, Xu C, Poh CL (2015) Diffusion weighted magnetic resonance imaging and its recent trend-a survey. Quant Imag Med Surg 5(3):407–422
Zhang J, Jones MV, McMahon MT, Mori S, Calabresi PA (2012) In vivo and ex vivo diffusion tensor imaging of cuprizone-induced demyelination in the mouse corpus callosum. Magn Reson Med 67:750–759
Ryutaro Y, Junichi H, Yoshifumi A, Fumiko S, Keitaro Y, Yuji K, Hideyuki O, Kenji FT (2018) Quantitative temporal changes in DTI values coupled with histological properties in cuprizone-induced demyelination and remyelination. Neurochem Intern 119:151–158
Liang Y, Ye AQ, Chen W, Gatto RG, Colon-Perez L, Mareci TH, Magin RL (2016) A fractal derivative model for the characterization of anomalous diffusion in magnetic resonance imaging. Commun Nonlinear Sci Numer Simul 39:529–537
Ingo C, Sui Y, Chen Y, Parrish TB, Webb AG, Ronen I (2015) Parsimonious continuous time random walk models and kurtosis for diffusion in magnetic resonance of biological tissue. Front Phys 3:11
Hrabe J, Hrabetova S, Segeth K (2004) A model of effective diffusion and tortuosity in the extracellular space of the brain. Biophys J 87(3):1606–1617
Sui Y, Wang H, Liu G, Damen FW, Wanamaker C, Li Y, Zhou XJ (2015) Differentiation of low- and high-grade pediatric brain tumors with high b-value diffusion-weighted MR imaging and a fractional order calculus model. Radiology 277(2):489–496
Acknowledgements
This work was support provided by grants from the National Center for Advancing Translational Science grant (NCATS TLTR000049 to AY), NIH DC02058 (to AL), CHDI (#A-11872; to GM and SB), NIH R21NS096642 (to GM). A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement no. DMR-1157490 and the State of Florida. In addition, we would like to thank Mr. Dan Plant for his input and assistance in the design and experimental set up for this project, as well as Dr. Carson Ingo for his assistance in fitting the data to the Mittag–Leffler function.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors of this manuscript have no conflicts of interest to report.
Ethical approval
Experiments were performed under protocols approved by the Animal Care Committee at the University of illinois in Chicago.
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gatto, R.G., Ye, A.Q., Colon-Perez, L. et al. Detection of axonal degeneration in a mouse model of Huntington’s disease: comparison between diffusion tensor imaging and anomalous diffusion metrics. Magn Reson Mater Phy 32, 461–471 (2019). https://doi.org/10.1007/s10334-019-00742-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-019-00742-6