Abstract
Object
Metabolite changes in an experimental lesion in the rat cortex and in the contralateral hemisphere after the intravenous administration of mesenchymal stem cells (MSCs) were assessed by proton MR spectroscopy to verify the impact of the cell treatment on the brain tissue.
Materials and methods
Wistar rats with a photochemical cortical lesion and transplanted MSCs or sham transplanted rats were examined. Proton spectra were obtained from the lesion and from the contralateral cortex.
Results
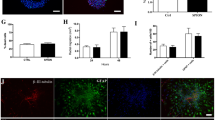
Magnetic resonance spectroscopy revealed a gradual recovery of the damaged tissue; however, we found no significant differences in metabolite concentrations in the lesioned hemisphere between treated and untreated animals. Higher concentrations of glutamate and N-acetyl aspartate were found in the contralateral hemisphere in cell-treated animals compared to untreated ones. Lesioned animals showed neurogenesis in the contralateral hemisphere; the number of newly generated cells in stem cell-treated animals was 50% higher than those observed in untreated animals.
Conclusion
No direct impact of cell transplantation was observed in the lesion. However, changes in the contralateral hemisphere suggest that the transplanted MSCs might stimulate repair processes and plasticity resulting in the generation of newborn cells, which might enable the faster adoption of the damaged tissue’s function by healthy tissue.
Similar content being viewed by others
References
Bjorklund A, Lindvall O (2000) Cell replacement therapies for central nervous system disorders. Nat Neurosci 3: 537–544
Ourednik V, Ourednik J, Park KI, Snyder EY (1999) Neural stem cells—a versatile tool for cell replacement and gene therapy in the central nervous system. Clin Genet 56: 267–278
Svendsen CN, Smith AG (1999) New prospects for human stem-cell therapy in the nervous system. Trends Neurosci 22: 357–364
Jendelová P, Herynek V, DeCroos J, Glogarová K, Andersson B, Hájek M, Syková E (2003) Imaging the fate of implanted bone marrow stromal cells labeled with superparamagnetic nanoparticles. Magn Reson Med 50: 767–776
Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71–74
Kopen GC, Prockop DJ, Phinney DG (1999) Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 96: 10711–10716
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 174(1): 11–20
Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M (2001) Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport 12: 559–563
Wu J, Sun Z, Sun H, Wu J, Weisel RD, Keating A, Li Z, Feng Z, Li RK (2008) Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant 16(10): 993–1005
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H (2005) Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther 11(1): 96–104
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98(5): 1076–1084
Norman AB, Thomas SR, Pratt RG, Lu SY, Norgren RB (1992) Magnetic resonance imaging of neural transplants in rat brain using a superparamagnetic contrast agent. Brain Res 594: 279–283
Hawrylak N, Ghosh P, Broadus J, Schlueter C, Greenough WT, Lauterbur PC (1993) Nuclear magnetic resonance (NMR) imaging of iron oxide-labeled neural transplants. Exp Neurol 121: 81–92
Shen T, Weissleder R, Papisov M, Bogdanov AJ, Brady TJ (1993) Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med 29: 599–604
Bulte JW, Brooks RA, Moskowitz BM, Bryant LHJ, Frank JA (1998) T1 and T2 relaxometry of monocrystalline iron oxide nanoparticles (MION-46L): theory and experiment. Acad Radiol 5: S137–S140
Bulte JW, Brooks RA, Moskowitz BM, Bryant LHJ, Frank JA (1999) Relaxometry and magnetometry of the MR contrast agent MION-46L. Magn Reson Med 42: 379–384
Bulte JW, Zhang SC, van Gelderen P, Herynek V, Jordan EK, Duncan ID, Frank JA (1999) Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci USA 96: 15256–15261
Jendelová P, Herynek V, Urdzíková L, Glogarová K, Kroupová J, Bryja V, Andersson B, Burian M, Hájek M, Syková E (2004) Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res 76(2): 232–243
Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD (1985) Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol 17: 497–504
Tkác I, Starcuk Z, Choi IY, Gruetter R (1999) In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 41: 649–656
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30: 672–679
Mahmood A, Lu D, Chopp M (2004) Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery 55: 1185–1193
Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M (2008) Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke 39(9): 2571–2577
Eaves CJ, Cashman JD, Kay RJ, Dougherty GJ, Otsuka T, Gaboury LA, Hogge DE, Lansdorp PM, Eaves AC, Humphries RK (1991) Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. II. Analysis of positive and negative regulators produced by stromal cells within the adherent layer. Blood 78: 110–117
Maysinger D, Berezovskaya O, Fedoroff S (1996) The hematopoietic cytokine colony stimulating factor 1 is also a growth factor in the CNS: (II). Microencapsulated CSF-1 and LM-10 cells as delivery systems. Exp Neurol 141: 47–56
Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, Chen J, Xu Y, Gautam S, Mahmood A, Chopp M (2002) Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res 69(5): 687–691
Urenjak J, Williams SR, Gadian DG, Noble M (1992) Specific expression of N-acetyl aspartate in neurons, oligodendrocyte- type-2 astrocyte progenitors and immature oligodendrocytes in vitro. J Neurochem 59: 55–61
Baslow MH (2002) Evidence supporting a role for N-acetyl-L-aspartate as molecular water pump in myelinated neurons in the central nervous system: an analytical review. Neurochem Int 40: 295–300
Baslow MH (2003) N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res 28: 941–953
Demougeot C, Marie C, Giroud M, Beley A (2004) N-acetylaspartate: a literature review of animal research on brain ischaemia. J Neurochem 90: 776–783
Al-Samsam RH, Alessandri B, Bullock R (2000) Extracellular N-acetyl-aspartate as a biochemical marker of the severity of neuronal damage following experimental acute traumatic brain injury. J Neurotrauma 17(1): 31–39
Puri BK, Smith HC, Cox IJ, Sargentoni J, Savic G, Maskill DW, Frankel HL, Ellaway PH, Davey NJ (1998) The human motor cortex after incomplete spinal cord injury: an investigation using proton magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry 65(5): 748–754
Likavčanová K, Urdzíková L, Hájek M, Syková E (2008) Metabolic changes in the thalamus after spinal cord injury followed by proton magnetic resonance spectroscopy. Magn Reson Med 59(3): 499–506
Escartin C, Valette J, Lebon V, Bonvento G (2006) Neuron– astrocyte interactions in the regulation of brain energy metabolism: a focus on NMR spectroscopy. J Neurochem 99: 393–401
Horner PJ, Thallmair M, Gage FH (2002) Defining the NG2-expressing cell of the adult CNS. J Neurocytol 31: 469–480
Levine JM, Reynolds R (1999) Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelinization. Exp Neurol 160: 333–347
McTigue DM, Wei P, Stokes BT (2001) Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci 21: 3392–3400
Hampton DW, Rhodes KE, Zhao C, Franklin RJ, Fawcett JW (2004) The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience 127: 813–820
Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V (2003) Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 161: 169–186
Verkhratsky A, Hettenmann H (1996) Calcium signaling in glial cells. Trends Neurosci 19: 346–352
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22: 208–215
Haydon P (2001) Glia. Listening and talking to the synapse. Nat Rev Neurosci 2: 185–193
Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signaling. Nature 369: 744–747
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herynek, V., Růžičková, K., Jendelová, P. et al. Metabolic changes in the rat brain after a photochemical lesion treated by stem cell transplantation assessed by 1H MRS. Magn Reson Mater Phy 22, 211–220 (2009). https://doi.org/10.1007/s10334-009-0166-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-009-0166-2