Abstract
Abstract
Objective
To evaluate the expression of p16INK4A gene in ovarian cancer and analyze the relation between this alteration and the promoter methylation of p16INK4A DNA.
Methods
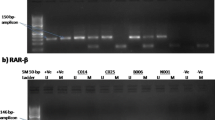
Seven ovarian cancer cell lines and eighteen ovarian cancer specimens were selected for the study. Genomic DNA and RNA were extracted from fresh tissues and cell lines, DNA was treated with sodium bisulfite and then analyzed with methylation-specific PCR (MSP) to detect p16INK4A methylation. The expression of p16INK4A mRNA was detected by reverse transcription-polymerase chain reaction (RT-PCR). In addition, the proliferation of methylated cell lines before and after treatment of demethylating agent 5-Aza-2′-deoxycytidine (5-ADC) was examined with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay in vivo.
Results
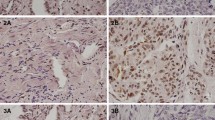
Compared with the control, the expression of p16INK4A mRNA decreased significantly or absolutely defaulted in 10 of 18 (55.56%) ovarian cancer specimens and 71.4% (5/7) ovarian cancer cell lines (P<0.05), and the expression of p16INK4A protein also decreased (P<0.05). The decrease of p16INK4A was due, in part, to p16INK4A methylation, which was found in the first exon of three cell lines and six ovarian cancer specimens and the rate was 42.86% and 33.33% in ovarian cancer cell lines and specimens respectively. All the methylated cells and tissues showed expression defect of p16INK4A, but the treatment of 5-ADC reactivated the expression of p16INK4A in methylated cells and decreased the proliferation of tumor cells in vitro and in vivo.
Conclusion
The expression defect of p16INK4A gene possibly has an important role in the development of ovarian cancer, and this alteration is due, in part, to the methylation of the first exon in p16INK4A.
Similar content being viewed by others
References
Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature, 1993, 366: 704–707.
Kamb A, Gruis NA, Weaver-Feldhaus J, et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science, 1994, 264: 436–440.
Nobori T, Miura K, Wu DJ, et al. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature, 1994, 368: 753–756.
Harper JW, Elledge SJ. Cdk inhibitors in development and cancer. Curr Opin Genet Dev, 1996, 6: 56–64.
Miller CW, Aslo A, Campbell MJ, et al. Alterations of the p15, p16, and p18 genes in osteosarcoma. Cancer Genet Cytogenet, 1996, 86: 136–142.
Shapiro GI, Rollins BJ. p16INK4A as a human tumor suppressor. Biochim Biophys Acta, 1996, 1242: 165–169.
Liggett WH Jr, Sidransky D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol, 1998, 16: 1197–1206.
Serrano M. The INK4a/ARF locus in murine tumorigenesis. Carcinogenesis, 2000, 21: 865–869.
Rocco JW, Sidransky D. p16 (MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res, 2001, 264: 42–55.
Kusume T, Tsuda H, Kawabata M, et al. The p16-cyclin D1/CDK4-pRb pathway and clinical outcome in epithelial ovarian cancer. Clin Cancer Res, 1999, 5: 4152–4157.
Sui L, Dong Y, Ohno M, et al. Inverse expression of Cdk4 and p16 in epithelial ovarian tumors. Gynecol Oncol, 2000, 79: 230–237.
Havrilesky LJ, Alvarez AA, Whitaker RS, et al. Loss of expression of the p16 tumor suppressor gene is more frequent in advanced ovarian cancers lacking p53 mutations. Gynecol Oncol, 2001, 83: 491–500.
Ichikawa Y, Yoshida S, Koyama Y, et al. Inactivation of p16/CDKN2 and p15/MTS2 genes in different histological types and clinical stages of primary ovarian tumors. Int J Cancer, 1996, 69: 466–470.
Schuyer M, van Staveren IL, Klijn JG, et al. Sporadic CDKN2 (MTS1/p16ink4) gene alterations in human ovarian tumours. Br J Cancer, 1996, 74: 1069–1073.
Wong YF, Chung TK, Cheung TH, et al. Methylation of p16INK4A in primary genecologic malignancy. Cancer Lett, 1999, 136: 231–235.
Loughran O, Malliri A, Owens D, et al. Association of CDKN2A/p16INK4A with human head and neck keratinocyte replicative senescence: relationship of dysfunction to immortality and neoplasia. Oncogene, 1996, 13: 561–568.
Costello JF, Berger MS, Huang HS, et al. Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res, 1996, 56: 2405–2410.
Lo KW, Cheung ST, Leung SF, et al. Hypermethylation of the p16 gene in nasopharyngeal carcinoma. Cancer Res, 1996, 56: 2721–2725.
Sambrook J, Russell DW, edited. Jin DY, translated. Molecular cloning: a laboratory manual. 2rd ed. Beijing: Science Press, 1996. 870–897.
Bird A. DNA methylation de novo. Science, 1999, 286: 2287–2288.
Gonzalez-Zulueta M, Bender CM, Yang AS, et al. Methylation of the 5′ CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res, 1995, 55: 4531–4535.
Katsaros D, Cho W, Singal R, et al. Methylation of tumor suppressor gene p16 and prognosis of epithelial ovarian cancer. Gynecol Oncol, 2004, 94: 685–692.
Momparler RL, Ayoub J. Potential of 5-aza-2′-deoxycytidine (Decitabine) a potent inhibitor of DNA methylation for therapy of advanced non-small cell lung cancer. Lung Cancer, 2001, 34Suppl 4: S111–115.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grant from the National Natural Science Foundation of China (No. 30070786) as well as Scientific and Technological Development Plan of Hubei Province of China
Rights and permissions
About this article
Cite this article
Li, M., Dong, W., Li, X. et al. The relationship between methylation and expression defect of tumor suppressor gene p16INK4A in epithelial ovarian cancer. Chinese German J Clin Oncol 5, 204–208 (2006). https://doi.org/10.1007/s10330-005-0428-z
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10330-005-0428-z