Abstract
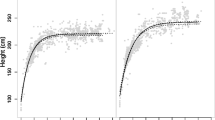
Measuring variations in body mass is necessary to gain a deeper understanding of the evolution of life-history patterns, and it provides information on the timing of sexual maturity and the development of sexual dimorphism. In this study, we collected longitudinal data on body mass from infancy to adulthood in a captive population of Tonkean macaques (Macaca tonkeana). Tests to evaluate whether social group, maternal age, and dominance rank influenced growth rates showed that they had no significant effect. We investigated the timing and magnitude of breaking points in the growth paths of males and females, and checked whether these breaking points could correspond to specific reproductive and morphological developmental events. We found that male and female Tonkean macaques have roughly equivalent body masses until around the age of four, when males go through an adolescent growth spurt and females continue to grow at a constant rate. Males not only grow faster than females, but they also continue to grow for nearly one and a half years after females have attained their full body mass. Growth rate differences account for approximately two-thirds of the body mass sexual dimorphism; only the remaining third results from continued male growth beyond the age where full body mass is reached in females. We also discovered remarkable correspondences between the timing of testicular enlargement and the adolescent growth spurt in males, and between dental development and slowdown breaking points in both sexes.

Similar content being viewed by others
References
Altmann J, Alberts SC (1987) Body mass and growth rates in a wild primate population. Oecologia 72:15–20
Altmann J, Alberts SC (2005) Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol 57:490–501
Amici F, Call J, Aureli F (2012) A version to violation of expectations of food distribution: the role of social tolerance and relative dominance in seven primate species. Behaviour 149:345–368
Aujard F, Heistermann M, Thierry B, Hodges JK (1998) Functional significance of behavioral, morphological and endocrine correlates across the ovarian cycle in semi-free ranging female Tonkean macaques. Am J Primatol 46:285–309
Bercovitch FB (1993) Dominance rank and reproductive maturation in male rhesus macaques. J Reprod Fertil 99:113–120
Bercovitch FB (2000) Behavioral ecology and socioendocrinology of reproductive maturation in cercopithecine monkeys. In: Whitehead PF, Jolly CJ (eds) Old World monkeys. Cambridge University Press, Cambridge, pp 298–320
Bernstein RM, Setchell JM, Verrier D, Knapp LA (2012) Maternal effects and the endocrine regulation of mandrill growth. Am J Primatol 74:890–900
Blackwelder TL, Golub MS (1996) Pubertal weight gain in female rhesus macaques. Am J Phys Anthropol 99:449–454
Bowman JE, Lee PC (1995) Growth and threshold weaning weights among captive rhesus macaques. Am J Phys Anthropol 96:159–175
Brizzee KR, Dunlap WP (1986) Growth. In: Dukelow WR, Erwin J (eds) Comparative primate biology: reproduction and development, vol 3. Alan R. Liss, New York, pp 363–413
Cheverud JM, Wilson P, Dittus WPJ (1992) Primate population studies at Polonnaruwa. III. Somatometric growth in a natural population of toque macaques (Macaca sinica). J Human Evol 23:51–77
Cooper MA, Chaitra MS, Singh M (2004) Effect of dominance, reproductive state, and group size on body mass in Macaca radiata. Int J Primatol 25:165–178
De Marco A, Sanna A, Cozzolino R, Thierry B (2014) The function of greeting interactions in male Tonkean macaques. Am J Primatol 76:989–998
Dixson AF, Nevison CM (1997) The socioendocrinology of adolescent development in male rhesus monkeys (Macaca mulatta). Horm Behav 31:126–135
Gaulin SJC, Sailer LD (1984) Sexual dimorphism in weight among the primates: the relative importance of allometry and sexual selection. Int J Primatol 5:515–535
Glassman DM, Coelho AM, Carey KD, Bramblett CA (1984) Weight growth in savannah baboons: a longitudinal-study from birth to adulthood. Growth 48:425–433
Glick BB (1979) Testicular size, testosterone level, and body weight in male Macaca radiata: maturational and seasonal effects. Folia Primatol 32:268–289
Gore MA (1993) Effects of food distribution on foraging competition in rhesus monkeys, Macaca mulatta, and hamadryas baboons, Papio hamadryas. Anim Behav 45:773–786
Hamada Y (1991) Postnatal growth of the Japanese macaques (Macaca fuscata) from birth to full maturity. In: Ehara A, Kimura T, Takenaka O, Iwamoto M (eds) Primatology today. Elsevier, Amsterdam, pp 451–454
Hamada Y, Hayakawa S, Suzuki J, Ohkura S (1999) Adolescent growth and development in Japanese macaques (Macaca fuscata): punctuated adolescent growth spurt by season. Primates 40:439–452
Herrenschmidt N (1977) Semi-free breeding of tropical Celebes macaques (Macaca tonkeana) in a continental European climate. J Med Primatol 6:58–65
Johnson SE (2003) Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. Am J Phys Anthropol 120:83–98
Lee PC (1999) Comparative ecology of postnatal growth and weaning among haplorhine primates. In: Lee PC (ed) Comparative primate socioecology. Cambridge University Press, Cambridge, pp 111–139
Leigh SR (1992) Patterns of variation in the ontogeny of primate body size dimorphism. J Human Evol 23:27–50
Leigh SR (1996) Evolution of human growth spurts. Am J Phys Anthropol 101:455–474
Moses LE, Gale LC, Altmann J (1992) Methods for analysis of unbalanced, longitudinal, growth data. Am J Primatol 28:49–59
Plavcan JM (2001) Sexual dimorphism in primate evolution. Yrbk Phys Anthropol 44:25–53
Plavcan JM (2011) Understanding dimorphism as a function of changes in male and female traits. Evol Anthropol 20:143–155
Schillaci MA, Stallmann R (2005) Ontogeny and sexual dimorphism in a population of booted macaques (Macaca ochreata). J Zool Lond 267:19–29
Schillaci MA, Froehlich JW, Supriatna J (2007a) Growth and sexual dimorphism in a population of hybrid macaques. J Zool 271:328–343
Schillaci MA, Jones-Engel L, Lee BPYH, Fuentes A, Aggimarangsee N, Engel GA, Sutthipat T (2007b) Morphology and somatometric growth of long-tailed macaques (Macaca fascicularis fascicularis) in Singapore. Biol J Lin Soc 92:675–694
Setchell JM, Lee PC, Wickings EJ, Dixson AF (2001) Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx). Am J Phys Anthropol 115:349–360
Setchell JM, Wickings EJ, Setchell JM, Wickings EJ, Knapp LA (2006) Life history in male mandrills (Mandrillus sphinx): physical development, dominance rank, and group association. Am J Phys Anthropol 131:498–510
Shea BT (1986) Ontogenetic approaches to sexual dimorphism in anthropoids. Hum Evol 1:97–110
Smith RJ, Jungers WL (1997) Body mass in comparative primatology. J Hum Evol 32:523–559
Spiegel A (1985) Postnatal growth of Macaca fascicularis born in captivity. Prim Rep 13:1–55
Stahl D, Kaumanns W (2003) Food competition in captive female sooty mangabeys (Cercocebus torquatus atys). Primates 44:203–216
Stephens SBZ, Wallen K (2013) Environmental and social influences on neuroendocrine puberty and behavior in macaques and other nonhuman primates. Horm Behav 64:226–239
Thierry B (2007) Unity in diversity: lessons from macaque societies. Evol Anthropol 16:224–238
Thierry B (2011) The macaques: a double-layered social organization. In: Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM (eds) Primates in perspective, 2nd edn. Oxford University Press, New York, pp 229–241
Thierry B, Anderson JR, Demaria C, Desportes C, Petit O (1994) Tonkean macaque behaviour from the perspective of the evolution of Sulawesi macaques. In: Roeder JJ, Thierry B, Anderson JR, Herrenschmidt N (eds) Current primatology: social development, learning and behaviour, vol 2. Université Louis Pasteur, Strasbourg, pp 103–117
Thierry B, Heistermann M, Aujard F, Hodges JK (1996) Long-term data on basic reproductive parameters and evaluation of endocrine, morphological and behavioral measures for monitoring reproductive status in a group of semi-free ranging Tonkean macaques (Macaca tonkeana). Am J Primatol 39:47–62
Vanćata V, Přívratský V, Zlámalová H, Vanćatová M, Mazura I (1999) A longitudinal study of ontogeny of Macaca mulatta. Var Evol 7:5–29
Zhao Q, Deng Z (1988) Macaca thibetana at Mt. Emei, China: I. A cross-sectional study of growth and development. Am J Primatol 16:251–260
Acknowledgments
We thank the managers and keepers of the Parco Faunistico di Piano dell’Abatino of Rieti for providing technical support. We are grateful to Faye Abbiate, Melissa Messinese and Elena Vero for their assistance during body mass measurements, and to Alessandro Giuliani for his statistical advice. We thank reviewers for their fruitful comments. This study followed all applicable international and national guidelines for the care and use of animals.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sanna, A., De Marco, A., Thierry, B. et al. Growth rates in a captive population of Tonkean macaques. Primates 56, 227–233 (2015). https://doi.org/10.1007/s10329-015-0465-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-015-0465-3