Abstract
The discovery of antibiotics remains one of the greatest achievements of the last century. Unfortunately, due to their overuse and misuse, the occurrence of resistance to antibiotics has become one of the most pressing dilemmas for human public health. Many sources of antibiotic resistance are known, including agriculture, animal husbandry, hospitals, sewage treatment plants, and potentially, cemetery soil. There is lack of sufficient evidence on the contribution of the cemetery soil in the introduction of antibiotic resistance into the environment; therefore, this issue needs to be explored. Here, we review the most important pathways for the transfer of antibiotic-resistant microorganisms and their genes and the main factors influencing the spreading efficiency. Unlike other studies on this subject, the article focuses on an area that seems to be overlooked, the cemetery soil environment. The presented data highlight the importance of cemetery soil in the spread of antibiotic-resistant microorganisms and their genes into the environment, which may help identify appropriate solutions to combat this problem more effectively. In addition, the review describes their potential importance in the escalation of the antibiotic resistance phenomenon, along with different methods of combating antimicrobial resistance.
Similar content being viewed by others
Introduction
Antibiotics are natural substances produced mostly by bacteria and fungi, able to inhibit or kill competing species. This discovery is one of the most important, medical achievements of the twentieth century (Russel 1977; Ribeiro da Cunha et al. 2019). Their inhibitory mechanism of action targets mainly the synthesis of the cell wall, disrupts the periplasmic membrane, and inhibits protein biosynthesis and DNA replication (Rushton 2015; Sriram et al. 2021). Unfortunately, their effectiveness is declining due to the growing problem of antibiotic resistance properties.
Antibiotic resistance properties are the mode of adaptivity formed in order to defend certain microorganisms against antimicrobial agents, existing for decades of natural evolutionary process. Antimicrobial resistance may also evolve through the mediation of antibiotic resistance genes via the horizontal gene transfer. In the case of the horizontal gene transfer, the transfer of resistance genes occurs by plasmids, by transformation, transduction, or conjugation (Lopatkin et al. 2016; Nicolaou and Rigol 2018).
The transformation is based on the acquisition of naked, outside the cell, DNA from the external environment by a competent cell, i.e., with the ability to transform. There are many species of bacteria with this trait, and most of these known so far occur naturally as a periodic physiological state (Chen and Dubnau 2004). Transduction and conjugation are based on the use of mobile genetic elements, including large plasmids and bacteriophages (Frost et al. 2005).
Transduction is the transfer of DNA from one cell to another using phage (Chen and Dubnau 2004). Two forms of transduction can be distinguished, general and specific. In the former, bacterial DNA is stuffed into the phage heads instead of its own genetic material. Any fragment of the genome can be packed in. Specific transduction is the result of an erroneous excision of a prophage from a specific site of integration. The resulting phage is a component of the actual phage’s DNA as well as the adjacent DNA from the bacterial genome. Stable inheritance of donor material occurs either through site-specific recombination mediated by phage integrase, or homologous recombination by bacterial recombinases (Thierauf et al. 2009).
Conjugation is considered to be the most troublesome of the horizontal gene transfer to spread antibiotic resistance among pathogens. The associated genetic element encodes proteins that allow it to be transferred to other cells, often including genes for antibiotic resistance and virulence factors (Graf et al. 2019). Acquired resistance due to the acquisition of resistance genes or the result of spontaneous mutations by bacteria is expressed by a number of mechanisms, such as modified antimicrobial target, enzymatic hydrolysis, efflux, and drug impermeability (Holmes et al. 2016; O'Neill 2016). However, due to increasing consumption and improper use of antibiotics, treatment of infections with antimicrobials is observed to be ineffective (de Ilurdoz et al. 2022; Salmanov et al. 2023). The most important sources of antibiotic-resistant microorganisms include agriculture (Li et al. 2011), animal husbandry (Campagnolo et al. 2002; Christian et al. 2003; Rushton 2015; Javid et al. 2016), aquaculture (Schar et al. 2020; Sriram et al. 2021; Thiang et al. 2021), hospitals and their pollutants (Rodriguez-Mozaz et al. 2015; Salmanov et al. 2023), wastewater treatment plants (Oliveira et al. 2021; Zieliński et al. 2021; de Ilurdoz et al. 2022) and cemeteries (Abia et al. 2018, 2019). However, cemetery soil has received little attention in terms of antibiotic resistance phenomena, although it appears to be a promising area.
Antibiotic resistance is one of the greatest challenges of the twenty-first century. That is why it is crucial to understand its mode of transfer mainly from antibiotic-resistant microorganisms and antibiotic resistance genes into the environment. Aspects such as unknown cause of death, the extent of microbial population present in and on the cadaver and their diversity, multiple gene transfer mechanisms and the high rate of mutation make it a reasonable assumption to consider cemetery soil as a possible reservoir of antimicrobial resistance.
Sources of antibiotics emission into the natural environment
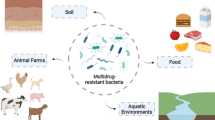
Transmission of drug-resistant microorganisms, and their genes, can occur via air, droplets, sex, feces, skin contact, contact with contaminated body fluids, water, food, and with participation of animal vectors (Jamrozik and Selgelid 2020). Regulatory gaps, or their absence and non-compliance with them, lead to the release of antibiotics into the environment (O'Neill 2016; Salmanov et al. 2023). This section outlines the most important sources of the spread of antibiotics into the environment, shown in Fig. 1.
Animal agriculture and aquaculture
Livestock as a part of an agriculture is one of the main markets for the use of antimicrobials (Rushton 2015; Sriram et al. 2021). In animal husbandry, antibacterials are used for therapeutic and for non-medical purposes. Non-medical use results from the desire to increase productivity and feed utilization while maintaining low production costs (Rushton 2015). Such practices, due to the growing misuse of antibiotics in animal husbandry, have significant impact on the escalation of antimicrobial resistance.
The FDA U.S. Food & Drug Administration (2021) reported the decline in domestic sales and distribution of medically important, i.e., important to human medicine, livestock antimicrobials. The drugs included in the report in terms of declining sales and distribution levels for food-producing animals for 2020, respectively, include: tetracyclines 66%, penicillin 13%, macrolides 7%, sulfonamides 5%, aminoglycosides 5%, lincosamides 2%, cephalosporins below 1% and fluoroquinolones below 1%. However, the authors pointed out that while interpreting, it should be considered that the presented data are limited to the estimation on sale and distribution of drugs for use in food-producing animals, not its actual usage. In addition, some of the agents can be used for other purposes than in animals for food production.
Campagnolo et al. (2002) studied swine and poultry manure waste lagoon and showed high concentrations of antibiotics and its residues (Table 1). All the analyzed samples revealed multiple classes of antimicrobials, tetracyclines, sulfonamides, β-lactams, macrolides and fluoroquinolones. The results indicated that in total, drugs residues reached almost 1 mg/l, i.e., summation of all antibacterials detected in a given sample, and individual antimicrobial agent usually exceeded 100 μg/l. This confirmed that at least a portion of the medicaments administered to swine are not absorbed by the animals and are excreted to the environment. Also, study of the dried liquid manure showed high concentrations of antibiotics at the soil surface, reaching 349.3 μg/kg of tetracycline and 1435 μg/kg of chlortetracycline (Table 1) (Hamscher et al. 2002; Javid et al. 2016). It is alarming because such aggregates can remain intact in soil. Consequently, bacteria are exposed to inhibitory levels of antibiotics, which can be toxic and reduce the biodiversity of the soil microbiome.
Christian et al. (2003) investigated the possibility of introducing antimicrobials and their residues through manure in fields, by surface runoff, leaching and drift. Drugs were detected in all the surface water samples (Table 1). Also, soil samples showed the presence of sulfadimidine, which was previously revealed in liquid manure, used as soil fertilizer (Table 1). The researchers have emphasized that sulfadimidine was found in the soil seven months after fertilization, which may imply a significant stability of some antibiotics in manure and soil. Li et al. (2011) proved that some drugs, i.e., chlortetracycline, sulfameter and quinolones have been detected in soils from vegetable crops at a concentration that may cause an ecotoxic effect, 100 μg/kg, according to the Steering Committee of the International Veterinary Committee for Harmonization (Table 1).
Also, Fang et al. (2018) proved the possibility of transmission and spread of antimicrobial residues, antibiotic resistance genes, human pathogenic bacteria and, what is more worrying, antibiotic-resistant human pathogenic bacteria from swine feedlot to the streams nearby, as well as agricultural soil, by means of sewage discharge and manure fertilization. The results showed significantly higher concentration of antibiotics residues in stream sediments and agricultural soils than in stream water (Table 1). The most frequently recorded antibiotic resistance genes in pig manure and stream sediments were those determining resistance to tetracyclines, while multidrug-resistance genes dominated in stream waters, similarly to agricultural soil (Table 2). The authors noted that although the amount of antibiotic resistance genes showed a steady decline along sewage and manure treatment channels, antibiotic resistance genes can still be transported from pig feed to nearby stream, sediments and greenhouse soil.
A similar phenomenon, i.e., leaching, may occur in cemetery soils. Bacteria, including those potentially resistant to antibiotics and their genes, after being released from the corpse, reach the soil, from where they can end up in watercourses, and then into the organisms of animals and humans. In addition, locating cemeteries on hills increases this risk. Climate change, which is conducive to more and more frequent floods, rainfall and snowmelt, makes this threat even higher.
In aquaculture, the use of antimicrobials is primarily for medicinal purposes. However, irrespective of the subject to be treated, efficacy is dose-dependent, which is a major problem in this case. Often, fish are given insufficient doses of the drugs. This may result from the use of counterfeit medicines and without the breeder's knowledge. Studies have shown that 48% of the pharmaceuticals analyzed were inauthentic, which meant that the active ingredient content was at least 20% lower than indicated on the label (Leung et al. 2020). The results of such standards are primarily ineffective therapies, and thus an increase in mortality among farm animals, economic losses, and the development of antibiotic-resistant microorganisms.
Schar et al. (2020) estimated that the worldwide use of antibiotics in humans and animals, both aquatic and terrestrial bred for food production will amount to 236,575 tons. Even though aquaculture accounts for only 5.7% of animal food production, it has the highest use of antibiotics per kg of biomass. Antimicrobial’s consumption in aquaculture is increasing faster than in meat and dairy products (Sriram et al. 2021). About 10,259 tons of antibiotics were allocated to this sector in 2017, and it is predicted that in 2030 it will reach 13,600 tons (Schar et al. 2020). Around 23 antibiotics have been identified in aquaculture on the Malaysian Peninsula, the most common were tetracyclines, sulfonamides and quinolones (Table 1), which is the evidence of the widespread use of antibacterial agents in local farms. Furthermore, genes for antimicrobial resistance have been revealed in over 90% of the sites, with the exception of sul3 (Thiang et al. 2021).
In addition, Schar et al. (2020) stressed out that it is worrying that the level of antibiotic use in selected aquatic animal species exceeds that of terrestrial animals and humans. It is also necessary to introduce changes in the law in the field of animal husbandry, e.g., ensuring animal welfare, limiting total drug consumption, reducing stress levels and improving hygiene (Nunan 2022). These studies highlighted the need to improve the quality of monitoring usage of antibiotics in aquaculture and livestock, with particular emphasis on the safety of water and ecosystems. A similar threat comes from the cemetery environment, where microorganisms resistant to antibiotics and their genes can spread into the environment through the surrounding watercourses and groundwater.
Hospitals and non-hospital care
Latest European Centre for Disease Prevention and Control (2021) report showed that between 2011 and 2020 in the European Union and European Economic Area there was tendency in total healthcare, both in community and hospital sector, in which the usage of antibacterials for systemic use dropped, indicating positive impact of coordinated and European Union wide measures. Unfortunately, when examining each of the health care sectors separately, only the community trend clearly weakened. In addition, an increase in the use of broad-spectrum antimicrobials has been seen. The authors emphasized that data included in the report cover the first year of the COVID-19 pandemic, which differed significantly from previous years, what could be caused by changes in behavior patterns in society and the organization of the health care system (European Centre for Disease Prevention and Control 2021).
Salmanov et al. (2023) focused on multi-drug-resistant organisms transmission from patient to patient, determined by healthcare workers and environment in Ukrainian hospitals. Study showed increasing tendency of multi-drug-resistant organisms occurrence. Particularly alarming was the high level of multi-drug-resistant organisms resistant to third generation cephalosporins and carbapenems in pathogenic Klebsiella pneumoniae and Escherichia coli and in carbapenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae. Moreover, in 37.4% of multi-drug-resistant organisms beta-lactamase genes were detected, including extended-spectrum beta-lactamases. Among extended-spectrum beta-lactamases, blaCTX-M, blaTEM and blaSHV types were the most common, with 41.7%, 35.7% and 33.9% detection, respectively. Detection rate of the extended-spectrum beta-lactamases-producing bacteria among isolates from healthcare-associated infected patients, environmental surfaces and healthcare workers—hands, gowns/gloves, was 35.9%, 34.1% and 37.5%, respectively (Table 2).
Similar research conducted by Rodriguez-Mozaz et al. (2015) examined the effectiveness of eliminating antimicrobials and antibiotic resistance genes from hospitals and integrated urban wastewater system by wastewater treatment plants and their levels in rivers receiving treated wastewater. Results showed that several antibiotics, such as ciprofloxacin and ofloxacin, were more prevalent in hospital than in wastewater treatment plants influents (Table 1). The findings also indicated that hospital effluent and wastewater treatment plants influent included the highest levels of copy counts of antibiotic-resistance genes—blaTEM, qnrS, ermB, sulI, and tetW (Table 2). Even though a noticeable decrease in copy numbers of these antibiotic-resistance genes in wastewater treatment plants effluent was noted, it was not consistent across all the investigated antibiotic-resistance genes. Moreover, the authors noticed that the receiving river was adversely impacted by the inadequate removal of antibiotics and antibiotic-resistance genes in the wastewater treatment plants since both emergent pollutants were present at higher concentrations in the waters downstream than in samples taken upstream from the discharge point. In contrary, Javid et al. (2016) did not report the presence of antibiotics—tetracyclines, in wastewater from a hospital wastewater treatment plant in Iran (Table 1). According to the authors, this is due to the discontinuation of its application to patients hospitalized in that region. However, the results from the remaining samples, from the municipal sewage treatment plant, clearly indicate the possibility of contamination of surface waters caused by overuse of antibacterials.
The shortage of the available of effective therapeutic agents exacerbates the increase in the rate of deaths caused by antimicrobial resistance (O'Neill 2016) and prevents the performance of many medical procedures, e.g., organ transplants, surgeries, cancer treatment or immunosuppression (World Health Organization 2018; Jamrozik and Selgelid 2020). In the context of direct antimicrobials ingestion, the lack of effective antibiotics leads to increasing difficulties in treating many diseases. Reduced therapeutic effectiveness concerns, inter alia, urinary tract infections, tuberculosis, sepsis, or gonorrhea in many places around the world (World Health Organization 2018). The increase in morbidity and mortality caused by drug resistance favors the expansion of antibiotic resistance from cemetery soils into the environment as with the growing population, the percentage of deaths caused by it will increase. Thus, despite the existence of other forms of burial, the number of traditional burials in cemeteries will increase proportionally, which will be a source of antibiotic-resistant microorganisms and its genes.
Sewage treatment plants and drinking water treatment plants
Wastewater treatment plants are another important source of the release of notable amounts of antibiotics, as well as antibiotic-resistant bacteria and antibiotic-resistance genes into the environment. Eventually, along with sewage, they end up in water bodies, e.g., seas, lakes or rivers (Javid et al. 2016; Osińska et al. 2020; Thakali et al. 2020). Research by Osińska et al. (2020) showed that despite a significant reduction in the concentrations of antibiotic-resistant bacteria and antibiotic-resistance genes as a result of wastewater treatment plants processes, excessive amounts continue to leak into the environment.
Zieliński et al. (2021) reported that biological and conventional wastewater treatment plants are one of the most important reservoirs of antibiotic-resistant microorganisms, their genes (Table 2) and mobile genetic elements. Inefficient treatment methods cause the components of treated sewage to get into surface waters. In addition to the release of antibiotics and their residues (Table 1) into the aquatic environment, it is also enriched with multi-drug resistance genes, with the participation of horizontal gene transfer. The article highlights the threats to sewage treatment plant workers as well. Respiratory swabs tests from wastewater treatment plants employees revealed that bioaerosols released in the activated sludge wastewater treatment plants can be a source of antibiotic-resistant microorganisms and antibiotic-resistance genes (Table 2) that threaten the health of the workers.
Rodriguez-Mozaz et al. (2015) showed that the level of wastewater treatment in the removal of antibiotic residues (Table 1) and antibiotic-resistance genes (Table 2) is insufficient, which poses a serious threat to humans and the environment. Research by Javid et al. (2016) also confirmed the presence of antibiotics in wastewater from a municipal sewage treatment plant (Table 1). Oliveira et al. (2021) studied sewage treatment plants—inflows and discharged effluent for the presence of bacteria resistant to carbapenems, both potentially environmental and pathogenic. The researchers found no acquired resistance genes among potentially environmental isolates, unlike the potentially pathogenic ones. The latter were characterized by a high level of acquired carbapenem resistance genes, as well as other classes of antibiotics, e.g., coding for resistance to β-lactams, aminoglycosides and fluoroquinolones. The most frequently detected carbapenem resistance gene among the identified isolates was blaKPC (Table 2). The authors pointed out the need to monitor the presence of an antibiotic-resistant bacteria in various environments and to develop and implement solutions that will increase the efficiency of wastewater treatment and safe discharge.
Recently, there has been growing interest in indirect methods of recording anthropogenic effects on the expansion of antibiotic-resistant bacteria. An example of such a solution is the use of bioindicators, i.e., class 1 integrase, which is becoming popular. Its presence has already been confirmed, inter alia, in human feces, sewage treatment plants, rivers, landfills or non-treated hospital wastewater (Stedtfeld et al. 2017). Thakali et al. (2020) attempted to determine the degree of removal of the class 1 integrase gene, intI1, and selected set of resistance genes, blaTEM, ermF, mecA and tetA, in two standard wastewater treatment plants in Louisiana, using chlorination. The authors concluded that chlorination-based treatment does not support the multiplication of antibiotic-resistant bacteria and their genes during the wastewater treatment process. However, it was noted that int1 is capable of surviving such processes.
The work of Xu et al. (2007) also draws attention to the importance of the anthropogenic contribution to the antimicrobial agents spread in the aquatic environment. A study indicated that the seawater of Victoria Harbor is not rich in antibiotics, as most of the analyzed ones were below the limit of quantification (Table 1). In contrast, Guangzhou's urban Pearl River region showed high concentrations of antibacterials, with significant diurnal variations depending on the high or low water season (Table 1). It is assumed that during the period of high water level in the river, the level of antibiotics was mainly determined by the cycles of wastewater production, which is conditioned by the rapid rate of refreshment. In turn, the period of low water level is regulated by tidal processes in the river.
Kim and Carlson (2007) proved that concentrations of antibiotics, i.e., tetracyclines, sulfonamides and macrolides, in water samples vary depending on the place and time of sampling from waters and sediments. Higher concentrations were recorded in winter, which may be justified by better stability of compounds at lower temperatures and lower flow capacity. In addition, higher levels of antimicrobials used by humans were observed downstream of wastewater treatment plants, and those used against animals in proximity to husbandry facilities. Moreover, higher concentrations of antibiotics were detected in sediment in comparison with water samples (Table 1). It was emphasized that sediments can be a source of these agents and, due to environmental changes, may release them into the water in future.
Petrovich et al. (2020) concerned the possibility of transferring antibiotic-resistant microorganisms and antibiotic-resistance genes to natural water reservoirs as a result of sewage discharge. Antibiotic-resistance genes were more abundant in hospital wastewater than in municipal wastewater, which was explained by the presence of human gut bacteria. Attention was also paid to the role of viruses able to determine the nature of bacterial community, by killing them, facilitating transduction and acting as prophages. Moreover, antibiotic-resistance genes, including those conferring resistance to clinically important antibiotics, were identified at all the sampling sites in the pilot-scale system, with only 16% of the total loss of antibiotic-resistance genes per genome equivalent between influent and effluent. Among the most common classes were those conditioning resistance to aminoglycosides, cephalosporins, macrolides, penams and tetracyclines. The authors observed that in hospital wastewater treatment system numerous types of antibiotic-resistance genes and viruses are preserved, which, because of wastewater discharge, may end up in the environment, causing a threat.
Antibiotic resistance studies in sewage treatment plants prove that there is no perfect method to eliminate antibiotic-resistant microorganisms and their genes, as well as the antibiotics themselves, of anthropogenic origin. In addition, the final place of release of the above-mentioned biological pollutants will be the cemetery soil. Despite the fact that there are fewer microorganisms in cemetery soil than in sewage treatment plants in terms of surface, their abundance and diversity are still high. In addition, due to the growing population, the number of traditional burials will increase.
Cemeteries are not subject to sewage treatment processes, which, together with non-compliance with hydrogeological conditions, will facilitate the release of leachates from the cemetery soil, ultimately intensifying the spread of antibiotic resistance to the environment.
Mentioned by Zieliński et al. (2021) the occupational risk of treatment plant workers also applies to employees of the funeral industry. When digging graves, carrying out exhumations and preparing the deceased for burial, pollutants will be released, including antibiotic-resistant microorganisms and their genes that pose a threat to workers.
Cemetery soil—a possible area
Despite growing interest in cremation, burying cadavers in graveyards remains one of the most common forms of burial in many countries (Gwenzi 2020; Kaźmierski 2020; Baum et al. 2021; Neckel et al. 2021). This may pose a potential sanitary and epidemiological threat (Lins et al. 2019; Egbimhanlu et al. 2020). Apart from uncontrolled use and misuse of antibiotics in agriculture and inappropriate medical practices, human cadavers and cemetery soil are prone to be excellent source of antibiotic-resistant microorganisms, especially bacteria. Bacteria are antibiotic-resistance genes generators, and due to their ability to mutate and penetrate groundwater and surface water resources, they can contribute to the escalation of the problem of antimicrobial resistance, with the participation of cemeteries.
A simplified scheme of the transfer of antibiotic-resistant bacteria and their genes from the cemetery soil to the environment is shown in Fig. 2. Studies revealed the presence of multi-drug resistance pathogenic bacteria in cemetery soil (Abia et al. 2019) and water samples (Table 2) collected from graveyards (Abia et al. 2018), both from the surface layers and from a depth of 2 m. Abia et al. (2018) investigated multi-drug resistance of Escherichia coli isolates from cemetery water samples, surface and borehole to eight selected antibiotics. About 87% of the isolates showed resistance to at least one of the agents. Streptomycin and trimethoprim were the most frequent drugs against which resistance was reported. Abia et al. (2019) noted greater microbiological diversity in the surface layers of the cemetery soil, whereas abundance in the number and functional classes of microorganisms responsible for human diseases, e.g., tuberculosis, Alzheimer's disease, pathways in cancer and Huntington's disease, was higher in deeper layers of soil. In addition, signatures related to antimicrobial resistance and the synthesis of strong antibiotics were identified. Moreover, one of the cemeteries—Maitland, was in direct contact with informal human settlements, thus indicating higher numbers of Escherichia coli from the surface water samples (Abia et al. 2018).
HGT—horizontal gene transfer
Sommer et al. (2009) investigated microflora resistance resources from saliva and feces of two healthy, unrelated people, restricted from taking antimicrobial agents for a year. The results of metagenomic analyses showed that majority of human microflora antibiotic resistance genes and pathogens were poorly related. They believed this might be caused by low availability or unprecedented flow of genes between human microbiome and their typical microbes. However, the functionality of genes in Escherichia coli has been demonstrated by the transformation. The authors think that there is some obstacle to gene transmission between the microbiome and pathogens, but certainly not due to functional incompatibility. It was indicated that the number of genes carrying antimicrobial resistance may over time contribute to the emergence of antimicrobial resistance among pathogenic microorganisms toward humans.
Kent et al. (2020) conducted experiments involving the possibility of spreading antibiotic resistance in the gut microflora and focused on the involvement of mobile genetic elements. Seven neutropenic patients with transplanted hematopoietic stem cells have received antibiotic therapy and were compared with two healthy people. The high growth of commensal antibiotic-resistance genes reservoir was observed in the gut, but no transfer of genes conferring resistance to antibiotics used in this area was noticed. Instead, the authors recorded the transfer of multi-drug resistance cassettes that confer resistance, among others, for beta-lactams and fluoroquinolones. They highlighted the importance of the human intestinal microflora, which, as it turns out, may be an active element of the mobile transfer of antibiotic-resistance genes.
Całkosiński et al. (2015) conducted a study of Polish cemeteries, based on the analysis of the composition of microflora and bacteria at different soil levels. The presence of pathogenic bacteria has been proven that can pose a threat to employees, as well as people visiting cemeteries. Examples of such pathogenic bacteria were Enterococcus spp.—80.6%, Bacillus spp.—77.4% and Escherichia coli—45.1%. The researchers noted no correlation between the sampling depth and bacterial growth, but the association with several differences in microbiota composition was observed. The need for more detailed research was highlighted, to determine the epidemiological nature of human infections and their possible connection with cemeteries, as such data are currently lacking.
During the decomposition of the cadaver, 0.4–0.6 l/kg of leachate is released in relation to the body weight. Depending on the abundance of pathogenic bacteria and viruses, groundwater could be potentially polluted, thus contributing to the spread of diseases among humans and animals (Żychowski and Bryndal 2015). In addition, nutrients released during the decomposition may regulate microbial diversity and soil functions, influencing the escalation rate of drug-resistant microorganisms (Abia et al. 2019).
According to Martinez (2009) human commensal and pathogenic bacteria present in the environment should be considered as its contaminants. The author observed that antibiotic-resistance genes need to cohabit same environment as human pathogens to enable gene transfer. It is facilitated by the increase in human population, and the lack of sufficient forms of wastewater-treatment poses a risk of antibiotic-resistance transfer to the natural environment. A similar relation can be observed in the environment of cemetery soil, where potentially human pathogens, including antibiotic-resistant ones, derived from cadavers may coexist in the same environment as natural soil microorganisms, facilitating the transfer of resistance genes.
Moreover, many cemeteries do not meet the requirements of the choice of establishment. It used to be a common practice to locate them near churches, mainly on hills, as well as within the vicinity of a water reservoirs. This can be one of the reasons for the increased risk of direct contamination of water reservoirs with leaks from graveyards. Currently, in most of the countries, locations selected for establishing cemeteries must meet sanitary safety requirements. Unfortunately, in the past, such security measures were not respected, which is why old cemeteries constantly pose a threat due to their current use. Żychowski and Bryndal (2015) suggested that old graveyards should be adapted to the current requirements. Moreover, the establishment and expansion of new ones should be proceeded by obtaining an appropriate environmental safety license. Rodrigues and Pacheco (2003) indicated the need to consider geological and hydrogeological factors, and other environmental factors, to protect groundwater.
An equally important factor is the concentration of antimicrobials released, assuming they could persist in the cadaver, into the water reservoirs, facilitating the acquisition of resistance by microorganisms. Research by Paíga and Delerue-Matos (2016) on groundwater samples from five cemeteries in Portugal confirmed the presence of pharmaceuticals—paracetamol, salicylic acid, ibuprofen, ketoprofen, nimesulide, carbamazepine, fluoxetine, and sertraline. Although antimicrobials were not identified, their occurrence cannot be excluded. The presence of pharmaceuticals proves that contamination from cemetery soils can affect surface waters pollution. Pacheco et al. (1991) dealt with the monitoring of the bacteriological quality of water samples in three Brazilian necropolises. They also confirmed the possibility of water contamination by cemeteries as well as their impact on groundwater. Rodrigues and Pacheco (2003) noted that the level of bacteriological contamination in the analyzed cemetery waters, namely total coli group and Escherichia coli, fecal streptococci, heterotrophic bacteria, at 22°C and 36°C, clostridium and proteolytic bacteria, was higher than these obtained from sampling points distant from graveyard, for two of three sites covered by the experiment.
Another important aspect is the method of keeping records of the causes of death. In Poland, they are at a low-quality level, referred as “garbage codes.” Residual data from death certificates do not allow for possible attempts to determine the cause of death and indicate the cause and effect relationship. This is an important issue because the information contained therein is necessary to assess the correctness of the methods used to treat the deceased and verify their effectiveness (Cierniak 2014). Having such data would be extremely valuable to determine the problem of antibiotic resistance, as it could contribute to more effective choice of treatment methods and the elimination of low-efficacy antimicrobials consumption. In Poland, there are no registries listing antibiotic resistance as the direct cause of death (Główny Urząd Statystyczny 2020), although it is a progressive problem on a global scale (O'Neill 2016). Such statistics would facilitate the risk assessment, e.g., to determine which strains of bacteria pose the greatest risk in a given country. Undoubtedly, this knowledge would contribute to improving the effectiveness of applied therapies and raising public awareness in the field of antibiotic resistance prevention.
In addition, the records of coffin types, i.e., materials used in them, may be useful for inferring the possible emission of toxic substances to the soil. Spongberg and Becks (2000) investigated the metal absorption rate of fine soils in a cemetery in Ohio. Atomic absorption spectrometry and inductively coupled plasma technique revealed numerous sources of contamination. Significant increases in concentrations were recorded for zinc, copper, lead, and iron, for both surface and in depth areas, especially below burial sites. The high increase in arsenic was explained by embalming and wood preservatives. Moreover, the importance of maintaining the general condition of the soil, groundwater and nearby surface water bodies was emphasized.
Mordhorst et al. (2022) studied the effects of heavy metals, e.g., zinc, lead, chromium, nickel, and copper, resulting from cremation and urns, on the environment. For this purpose, two cremated human remains, of a 74-year-old male and 70-year-old female and soil from six cemeteries with urn graves were analyzed. The researchers collected soil samples from the depth below burial point, after resting time and without urn burials as reference soil, i.e., the same area and depth of the cemetery. The results indicated significant variability, and release of heavy metals into cemetery soils were contributed by both, the ashes, and the raw materials from which the urns were made. They concluded that the differences may result from different occupational exposure, clothing, or the burial of the deceased. Significant differences were also observed in the soil samples from the buried urns. The accumulation of some metals, e.g., lead and tin in the soil was proportional to the degree of degradation of the urns, and the enrichment with copper, chromium, nickel, and iron was also discovered below but only slightly corroded.
The phenomenon of antibiotic resistance may be one of the causes of death, but due to the lack of research on this subject, assessing this probability is very difficult. Therefore, research on cemetery soil environment seems to be indispensable to assess the actual level of risk it poses to human and animal health, and the environmental contamination by antibiotic-resistant microorganisms. It is impossible to avoid the risk of the transmission of antibiotic-resistant microorganisms and their genes to the environment. The cemetery soil remains a poorly understood area and requires more extensive research, with comparative analysis of necropolises water reservoirs and groundwater. In addition, special attention should be taken when handling corpses during burial preparations. The available data showed numerous shortcomings. Sanitary aspects are often overlooked, which results in endangering the employees of funeral establishments and the environment.
Factors determining the Ôtransmission of antibiotic-resistant microorganisms and their genes to the natural environment
As antimicrobials are released into soil, such as graveyard soil, their environmental impact will depend on their persistence. The factors determining their functionality include sorption to organic particles and degradation or transformation (Cycoń et al. 2019). The existence of drug-resistant strains and genes in various environments is a common phenomenon. Antibiotic-resistant bacteria have been observed in different environments including Antarctica, sea, food, water or soil (Holmes et al. 2016). The factors facilitating or contributing to the emission of microorganisms resistant to antibiotics and antibiotic resistance genes, especially in necropolises, are soil persistence and acidity, antibiotic concentration, organic matter abundance, climatic conditions and ecosystem biodiversity and are characterized below.
One of the key factors affecting the potential for further transfer of antibiotic-resistant microorganisms and antibiotic-resistance genes in the environment are antibiotics concentration and soil properties (Shi et al. 2023). The type of soil determines the permeability of leachate from cemeteries to the environment. The low permeability of soils, e.g., clay, which favors leaching, the graveyards location on a hill and the proximity of water reservoirs, with the possible presence of antibiotic-resistance genes, pose a serious health risk.
A Cela-Dablanca et al. (2022) batch-type study on amoxicillin bioreduction in various soil types showed considerable variability. At the highest concentration of the antibiotics included in the study—50 μmol/l, the following adsorption values were recorded: 74.21–82.41% of the added antibiotic in forest soil, 68.31–92.56% in cornfield soil and 50.96–82.55% in vineyard soil. The authors observed that the greatest adsorption occurs in acidic soil, rich in organic matter and non-crystalline minerals. However, the total desorption of amoxicillin was less than 10%, the maximum value for forest soil was 6.5% and 16.9% for agricultural soil. It was concluded that soil acidity of pH below 5.5 and the abundance of organic matter and crystalline minerals promoted adsorption of amoxicillin. Conversely, weak desorption occurs less in acidic soil, rich in organic substances and non-crystalline minerals. This was considered to represent the risk of amoxicillin entering the soil, further transmitted to water bodies, and becoming part of the trophic chain (Cela-Dablanca et al. 2022).
Franklin et al. (2022) characterized the sorption and desorption properties of four antibiotics: sulfamethoxazole, trimethoprim, lincomycin and ofloxacin, commonly found in soil. The ratio of antibiotics, in water, to soil weight was 2:1. Antibiotics concentrations ranged from 1 to 24 μg/l, depending on the medication, based on the concentrations from wastewater-treatment plants. The sorption capacity was found to be higher when using a lower ratio of antibiotics, in enriched water, to soil. They concluded that ofloxacin and trimethoprim bind more firmly to soil, in contrast to sulfamethoxazole and lincomycin, which are more mobile due to lower sorption.
Zabłotni and Jaworski (2014) revealed the importance of antibiotics concentration. Subinhibitory levels of these compounds are present in soil, water or different organisms tissues as a result of extensive antimicrobials usage in both humans and animals. This may affect the activity of bacterial genes, causing changes in the level of transcription, increasing frequency of mutations and the transfer of mobile genetic elements through horizontal gene transfer, including between environmental strains.
Moreover, Li et al. (2019) noted the influence of climatic conditions. They focused on the wastewater as an important determinant contributing to the increase in the concentration of antimicrobials and their resistance genes in wetlands. Significantly higher concentrations of antibiotics were recorded in the winter, which was justified by the seasonal increase in their consumption by humans and the lower degree of removal and dilution caused by water temperature (− 0.5 to 13.5°C). In turn, in the summer, an increase in the number of antibiotic-resistance genes was observed, due to enhanced bacterial growth and their proliferation at higher temperatures. In addition, they detected the presence of selective pressure on antibiotic-resistance genes at sub-inhibitory concentrations in natural wetlands.
Antimicrobial agents also affect the biodiversity of soil microorganisms, while human activity, especially agricultural one, affects the presence of various groups of antibiotic-resistance genes in soil. Importantly, the ambiguity characterizing the results of the studies conducted on soil samples proved the complexity of the problem of the antimicrobial agents influence on soil microbial groups and it currently poses a considerable challenge. In addition, antibiotic residues in soil are characterized by a wide range of half-life values, which means that durability depends on numerous determinants, including physicochemical properties of the residue, soil specificity, as well as climatic conditions, such as temperature, rainfall, and humidity (Cycoń et al. 2019). Moreover, the presence of antibiotics in the soil may determine changes in its microbiome by inducing long-term selective pressure (Cerqueira et al. 2019). Besides, antimicrobials affect soil microorganisms by affecting their enzymatic properties and the ability to metabolize various carbon sources, and by regulating the total microbial biomass and the number of individual groups (Cycoń et al. 2019).
Lower microbial biodiversity of ecosystems, such as soil, may also positively correlate with the spread of drug-resistant strains. This variability is a natural defense mechanism against attacks of hostile microorganisms. The studies show that a decrease in the richness of soil microorganisms is a key factor that facilitates soil colonization by drug-resistant strains (Hamscher et al. 2002; Chen et al. 2019). Loss of soil biodiversity is a huge problem nowadays, as it threatens food security, human health, and pests control (Food and Agriculture Organization of the United Nations 2021). Thus, in view of these factors, it can be concluded that the deteriorating richness of soil microorganisms constitutes a significant impulse stimulating the multiplication of antibiotic-resistant strains.
How to combat antibiotic resistance?
Antimicrobials usage
As reported by Sriram et al. (2021) consumption of antimicrobials considered critical to human health, according to World Health Organization, increased globally by 91% between 2000 and 2015 and by 165% in low- and middle-income countries. The situation is similar for both terrestrial and aquatic animal husbandry and the use of antibiotics needs to be reduced. The penetration of an increasing number of antimicrobial agents into soils and waters poses a serious threat to all microorganisms inhabiting them (Cycoń et al. 2019). Without limiting their consumption of antibiotics, it is impossible to effectively fight antibiotic resistance to them.
Prevention
In underdeveloped countries, living conditions remain at a low level, e.g., lack of access to running water, even in hospitals, unequal access to medicines or non-compliance with hygiene rules is common. Misuse and unavailability of medications are a frequent problem, which favors the occurrence of improper treatment of infections, resulting in increase in antibiotic-resistant strains. Other factors include (a) limited access to antibiotics—untreated infections or treated with too low doses of the drug may favor the survival of resistant strains and their further spread, (b) excessive use of antimicrobials—beyond recommendations, favoring the increase in the number of drug-resistant strains (O'Neill 2016; Jamrozik and Selgelid 2020; Sriram et al. 2021). Fletcher (2015) drew attention to the essence of compliance with, inter alia, basic sanitary rules, food safety and secondary water and wastewater treatment. Investments to increase inoculation, improve drinking water quality, sanitation and implement antimicrobial management in healthcare settings can help reduce the effects of antimicrobial resistance worldwide (O'Neill 2016; Sriram et al. 2021). Also, improving sanitation and hygiene as a tool to eradicate infectious diseases remains an issue and is essential to combating antibiotic resistance.
Animal husbandry—improvement of the living conditions of farm animals
Reducing the use of antibiotics in the livestock sector remains one of the most important issues in stopping the spread of antibiotic-resistant microorganisms and antibiotic-resistance genes. In addition, it is necessary to improve animal husbandry conditions, lower densities and the use of higher quality food will help to reduce the number of infections, which will in turn reduce the need for antibiotics (McEwen et al. 2018; Jamrozik and Selgelid 2020). Nunan (2022) also pointed to the need to involve governments in improving animal husbandry conditions as well as improving monitoring in the area of antibiotic use and animal welfare by farmers. Furthermore, Guardabassi et al. (2004) suggested that giving up or partially reducing meat consumption can reduce the risk of antibiotic resistance in humans.
An indirect factor in the livestock sector is the unattractive remuneration of staff responsible for controlling the consumption of antibiotics. This results in the absence of such personnel or presence of unskilled staff, favoring the collection of low-quality or missing data on antimicrobial consumption, which makes it difficult to reliably assess the scale of the problem and response options (O'Neill 2016; Najwyższa Izba Kontroli 2019). The solution is to raise wages in this sector to be able to hire qualified staff.
Global awareness and vaccination programs
Global awareness of the public is essential in the fight against antibiotic resistance. Therefore, educating medical staff, politicians as well as all people indirectly related to this area is necessary to eliminate the problem. For this purpose, educational activities must be launched such as trainings, conferences, symposia, workshops, thematic events, promoting proper use of antimicrobials, consequences of overuse and further threats resulting from non-compliance with good practices (World Organisation for Animal 2016; O'Neill 2016; Najwyższa Izba Kontroli 2019). On an international scale, multidisciplinary in nature, the One Health plan has been established and involves three key organizations—World Health Organization, Food and Agriculture Organization of the United Nations and World Organisation for Animal. The cooperation covers various sectors—medicine, veterinary medicine, animal husbandry, agriculture, financial resources and the environment, whose common goal is to fight antibiotic resistance (World Health Organization 2015; World Organisation for Animal 2016).
Another strategic element with a global reach is the increase in vaccination of people and animals. Vaccination programs have the potential to eliminate antibiotics usage in future (World Health Organization 2018; Gajewska et al. 2020; Jamrozik and Selgelid 2020; Malchrzak and Mastelarz-Migas 2021). An example of the positive impact is the pneumococcal vaccination. This is one of the most effective methods of reducing pneumococcal diseases, which has also been observed in Poland. In the vaccination program in Kielce, a decrease in the number of fatalities was observed: in children of up to 2 years of age by 82.9% and in people over 65 years of age by 43.5% (Malchrzak and Mastelarz-Migas 2021) was observed. Unfortunately, Sriram et al. (2021) reported that despite the existence of vaccines for many infectious diseases, due to low vaccination rates, insufficient quality drinking water and inadequate sanitation, the percentage of people susceptible to infections and dependent on antibiotics is still increasing.
Cemetery soil environment
The graveyard soil remains a poorly researched area in terms of antibiotic resistance. The available data suggest that necropolises pose a serious threat to human health, due to the possibility of antibiotic-resistant microorganisms and antibiotic-resistance genes transmission to environment (Abia et al. 2018, 2019; Gwenzi 2020). Studies showed that cemeteries can be a source of environmental pollution, e.g., heavy metals (Spongberg and Becks 2000; Abia et al. 2019; Mordhorst et al. 2022) or pharmaceuticals (Żychowski and Bryndal 2015; Fiedler et al. 2018). For this reason, it is important to undertake research in the field of cemetery soil to assess their actual contribution to the spread of antibiotic-resistant microorganisms and antibiotic-resistance genes. In addition, the regulations on the establishment and maintenance of cemeteries, burial of the dead and methods of dealing with corpses should be changed to reduce environmental pollution, with particular emphasis on the sanitary aspect, as well as the direct threat to funeral home employees (Żychowski and Bryndal 2015; Gwenzi 2020). An appropriate solution to increase sanitary safety would be to use of cremation, but unfortunately in many countries this is still a relatively rare practice.
Searching for new antibiotics and alternatives
A key element is to improve the effectiveness of commercially available antibiotics to which pathogens have become resistant (Lambert 2005). Various drug innovations may be the solution. One possibility is chemical modification, e.g., in the semi-synthesis process (Pawlowski et al. 2016). Examples of such are new generations of β-lactams, glycopeptides and aminoglycosides widely used in clinical practice. Another way to improve the functionality of available antibiotics is the use of adjuvants. Agents, i.e., aminoglycosides, despite their high effectiveness, have a limitation consisting in the need to use high concentrations of these preparations, which can be toxic to patients. The use of adjuvants allows to reduce the dose of the preparation while maintaining its effectiveness (Rosenberg et al. 2021). Thus, it proves the possibility of extending the usefulness of the antibiotics used without a sudden increase in the number of pathogens resistant to them.
An equally important is the search for new antibiotics, which in recent years has been slow and often ineffective. Mediocre benefits combined with high costs discourage the search for new antimicrobial agents. The situation is extremely dangerous, as exemplified by tuberculosis, which is resistant to almost all antibiotics available on the market. Without intensifying work on the development of new or alternative medicinal substances, people will become completely helpless (Founou et al. 2017; World Health Organization 2018; Jamrozik and Selgelid 2020). In addition, an indispensable element of counteracting drug resistance is efficient diagnostics for the treatment of both humans and animals (World Organisation for Animal 2016; O'Neill 2016; Najwyższa Izba Kontroli 2019) and the creation or expansion of databases (O'Neill 2016; Najwyższa Izba Kontroli 2019). This will reduce the unnecessary use of antibiotics, thus extending the useful life of those currently available.
Conclusion
The consequence of antibiotic resistance is more than 2.8 million cases of antibiotic resistance each year, of which more than 35,000 people die (Centers for Disease Control and Prevention (U.S.) 2019). There are already cases of resistance to first-line antibiotics. It applies to the pathogens causing HIV, malaria, and typhoid fever, and primarily affects low- and middle-income countries (Sriram et al. 2021). About 25,000 people die from antibiotic resistance in Europe every year (European Commission 2017) and around 700,000 people worldwide. Moreover, it was estimated that globally, by 2050, death from antibiotic resistance will reach ten million cases annually. Nevertheless, the above figures are an underestimate and in reality, may turn out to be much higher (O'Neill 2016). Also, healthcare costs are increasing as a result of ineffective therapies, more frequent medical interventions and longer hospitalizations in developing as well as developed countries.
Summarizing, the studies carried out so far suggest that cemetery soil might be a habitat for pathogenic bacteria resistant to antibiotics and multidrug resistant, as well as antibiotic-resistance genes (Abia et al. 2018, 2019; Gwenzi 2020). These microorganisms can spread further into the environment and transmit resistance, including to pathogens. Environmental conditions, soil type and water table depth determine the bacteriological quality of groundwater (Pacheco et al. 1991), which may affect the rate of spread of antimicrobial resistance in a graveyard environment. The location of cemeteries is relevant factor that may facilitate the spread of antibiotic-resistant microbes with their genes as well as other contaminants.
Despite the growing tendency to choose alternative forms of burial, burials in cemeteries still constitute a significant percentage. Moreover, given the ever-growing population, along with the increasing number of diseases caused by antibiotic resistance, graveyard soil may play an important role in the spread of antibiotic-resistant bacteria and their genes. Considering the aspects, i.e., the lack of studies on the causality of deaths, the multitude of microorganisms, especially bacteria present in and on corpses and their density, the numerous, unknown mechanisms of gene transfer and the high rate of mutation, treating the soil as a reservoir of antibiotic resistance seems to be a valid assumption. Limited knowledge in this area creates a need for further research into this specific environment. Significant efforts need to be made to bring us closer to solving the problem of antibiotic resistance, and cemetery soil research can contribute to this.
References
Abia ALK, Ubomba-Jaswa E, Schmidt C, Dippenaar MA (2018) Where did they come from—multi-drug resistant pathogenic Escherichia coli in a cemetery environment? Antibiotics. https://doi.org/10.3390/antibiotics7030073
Abia ALK, Alisoltani A, Ubomba-Jaswa E, Dippenaar MA (2019) Microbial life beyond the grave: 16S rRNA gene-based metagenomic analysis of bacteria diversity and their functional profiles in cemetery environments. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2018.11.302
Baum CA, Becegato VA, Lavnitcki L, Vilela PB, Duminelli EC, Becegato VR, Robazzo WS, Paulino AT (2021) Evaluation of soil contamination by heavy metals at public cemeteries in the municipality of Lages, southern Brazil. Eng Sanit Ambient. https://doi.org/10.1590/S1413-415220200030
Całkosiński I, Płoneczka-Janeczko K, Ostapska M, Dudek K, Gamian A, Rypuła K (2015) Microbiological analysis of necrosols collected from urban cemeteries in Poland. Biomed Res Int. https://doi.org/10.1155/2015/169573
Campagnolo ER, Johnson KR, Karpati A, Rubin CS, Kolpin DW, Meyer MT, Esteban JE, Currier RW, Smith K, Thu KM, McGeehin M (2002) Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci Total Environ. https://doi.org/10.1016/s0048-9697(02)00233-4
Cela-Dablanca R, Barreiro A, López LR, Santás-Miguel V, Arias-Estévez M, Núñez-Delgado A, Álvarez-Rodríguez E, Fernández-Sanjurjo MJ (2022) Relevance of sorption in bio-reduction of amoxicillin taking place in forest and crop soils. Environ Res. https://doi.org/10.1016/j.envres.2022.112753
Centers for Disease Control and Prevention (U.S.) (2019) Antibiotic resistance threats in the United States, 2019. https://stacks.cdc.gov/view/cdc/82532. Accessed 10 Feb 2023
Cerqueira F, Matamoros V, Bayona JM, Berendonk TU, Elsinga G, Hornstra LM, Piña B (2019) Antibiotic resistance gene distribution in agricultural fields and crops. A soil-to-food analysis. Environ Res. https://doi.org/10.1016/j.envres.2019.108608
Chen I, Dubnau D (2004) DNA uptake during bacterial transformation. Nat Rev Microbiol. https://doi.org/10.1038/nrmicro844
Chen QL, An XL, Zheng BX, Gillings M, Peñuelas J, Cui L, Su JQ, Zhu YG (2019) Loss of soil microbial diversity exacerbates spread of antibiotic resistance. Soil Ecol Lett. https://doi.org/10.1007/s42832-019-0011-0
Christian T, Schneider RJ, Färber HA, Skutlarek D, Meyer MT, Goldbach HE (2003) Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim Hydrobiol. https://doi.org/10.1002/aheh.200390014
Cierniak M (2014) Na co umarł pacjent—czyli co jest wpisywane na kartach zgonów? https://stat.gov.pl/obszary-tematyczne/ludnosc/statystyka-przyczyn-zgonow/na-co-umarl-pacjent-czyli-co-jest-wpisywane-na-kartach-zgonow-,1,1.html. Accessed 26 Feb 2023
Cycoń M, Mrozik A, Piotrowska-Seget Z (2019) Antibiotics in the soil environment—degradation and their impact on microbial activity and diversity. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00338
de Ilurdoz MS, Sadhwani JJ, Reboso JV (2022) Antibiotic removal processes from water & wastewater for the protection of the aquatic environment-a review. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2021.102474
Egbimhanlu AE, Sophia OD, Korede AS, Adenike OE, Adegboyega AO, Omonigho DE, Efeovbokhan EV (2020) contamination assessment of underground water around a cemetery: case study of Ayobo cemetery in Lagos, Nigeria. Int J Eng Res. https://doi.org/10.37624/ijert/13.6.2020.1283-1288
European Centre for Disease Prevention and Control (2021) Antimicrobial consumption in the EU/EEA (ESAC-Net)—annual epidemiological report 2020. https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2020. Accessed 15 Jan 2023
European Commission (2017) A European One Health action plan against antimicrobial resistance (AMR). https://ec.europa.eu/health/system/files/2020-01/amr_2017_action-plan_0.pdf. Accessed 27 Jan 2023
Fang H, Han L, Zhang H, Long Z, Cai L, Yu Y (2018) Dissemination of antibiotic resistance genes and human pathogenic bacteria from a pig feedlot to the surrounding stream and agricultural soils. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2018.05.066
FDA U.S. Food & Drug Administration (2021) 2020 Summary report on antimicrobials sold or distributed for use in food-producing animals. https://www.fda.gov/media/154820/download. Accessed 2 Feb 2023
Fiedler S, Dame T, Graw M (2018) Do cemeteries emit drugs? A case study from southern Germany. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-0757-9
Fletcher S (2015) Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ Health Prev Med. https://doi.org/10.1007/s12199-015-0468-0
Food and Agriculture Organization of the United Nations (2021) Keep soil alive, protect soil biodiversity. Global symposium on soil biodiversity, 19–22 April 2021—outcome document. https://www.fao.org/documents/card/ru/c/cb6005en/. Accessed 16 Feb 2023
Founou RC, Founou LL, Essack SY (2017) Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0189621
Franklin AM, Williams C, Andrews DM, Watson JE (2022) Sorption and desorption behavior of four antibiotics at concentrations simulating wastewater reuse in agricultural and forested soils. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.133038
Frost LS, Leplae R, Summers AO, Toussaint A (2005) Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol. https://doi.org/10.1038/nrmicro1235
Gajewska M, Goryński P, Paradowska-Stankiewicz I, Lewtak K, Piotrowicz M, Urban E, Cianciara D, Wysocki MJ, Książek A, Izurieta P (2020) Monitoring of community-acquired pneumonia hospitalisations before the introduction of pneumococcal conjugate vaccine into Polish National Immunisation Programme (2009–2016): A nationwide retrospective database analysis. Vaccine. https://doi.org/10.1016/j.vaccine.2019.10.031
Graf FE, Palm M, Warringer J, Farewell A (2019) Inhibiting conjugation as a tool in the fight against antibiotic resistance. Drug Dev Res. https://doi.org/10.1002/ddr.21457
Guardabassi L, Schwarz S, Lloyd DH (2004) Pet animals as reservoirs of antimicrobial resistant bacteria: review. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkh332
Gwenzi W (2020) The ‘thanato-resistome’-The funeral industry as a potential reservoir of antibiotic resistance: early insights and perspectives. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.141120
Hamscher G, Sczesny S, Höper H, Nau H (2002) Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal Chem. https://doi.org/10.1021/ac015588m
Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, Guerin PJ, Piddock LJ (2016) Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. https://doi.org/10.1016/S0140-6736(15)00473-0
Jamrozik E, Selgelid MJ (2020) Drug-resistant infection: causes, consequences, and responses. In: Jamrozik E, Selgelid MJ (eds) Ethics and drug resistance: collective responsibility for global public health. Springer, Cham. https://doi.org/10.1007/978-3-030-27874-8_1
Javid A, Mesdaghinia A, Nasseri S, Mahvi AH, Alimohammadi M, Gharibi H (2016) Assessment of tetracycline contamination in surface and groundwater resources proximal to animal farming houses in Tehran, Iran. J Environ Health Sci Eng. https://doi.org/10.1186/s40201-016-0245-z
Kaźmierski P (2020) Cmentarze, łąki pamięci oraz grzebowiska—nowe formy pochówku wyzwaniem dla polskiego prawa funeralnego. In: Staniszewska PH, Aszkiełowicz P (eds) Prawo, religia, wyznanie: Na styku normatywizmu i moralności. Wydawnictwo Think & Make, Warszawa, pp 129–146
Kent AG, Vill AC, Shi Q, Satlin MJ, Brito IL (2020) Widespread transfer of mobile antibiotic resistance genes within individual gut microbiomes revealed through bacterial Hi-C. Nat Commun. https://doi.org/10.1038/s41467-020-18164-7
Kim SC, Carlson K (2007) Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ Sci Technol. https://doi.org/10.1021/es060737+
Najwyższa Izba Kontroli. Departament Zdrowia (2019) Bezpieczeństwo pacjentów przy stosowaniu antybiotykoterapii w szpitalach. https://www.nik.gov.pl/kontrole/P/18/058/KZD/. Accessed 29 Nov 2022
Lambert PA (2005) Bacterial resistance to antibiotics: modified target sites. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2005.04.003
Leung KC, Huang Q, St-Hilaire S, Liu H, Zheng X, Cheung KB, Zwetsloot IM (2020) Fraudulent antibiotic products on the market for aquaculture use. Prev Vet Med. https://doi.org/10.1016/j.prevetmed.2020.105052
Li YW, Wu XL, Mo CH, Tai YP, Huang XP, Xiang L (2011) Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearl River Delta area, southern China. J Agric Food Chem. https://doi.org/10.1021/jf1047578
Li S, Zhang R, Hu J, Shi W, Kuang Y, Guo X, Sun W (2019) Occurrence and removal of antibiotics and antibiotic resistance genes in natural and constructed riverine wetlands in Beijing, China. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.02.043
Lins EAM, Lins ASBM, Lins CMMS (2019) Negative environmental impacts generated by cemetery: Case study. Int J Adv Sci 4:16–19
Lopatkin AJ, Sysoeva TA, You L (2016) Dissecting the effects of antibiotics on horizontal gene transfer: analysis suggests a critical role of selection dynamics. BioEssays. https://doi.org/10.1002/bies.201600133
Malchrzak W, Mastalerz-Migas A (2021) epidemiologic benefits of pneumococcal vaccine introduction into preventive vaccination programs. Adv Exp Med Biol. https://doi.org/10.1007/5584_2020_589
Martinez JL (2009) Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Poll. https://doi.org/10.1016/j.envpol.2009.05.051
McEwen SA, Angulo FJ, Collignon PJ, Conly JM (2018) Unintended consequences associated with national-level restrictions on antimicrobial use in food producing animals. Lancet Planet Health. https://doi.org/10.1016/S2542-5196(18)30138-4
Mordhorst A, Zimmermann I, Fleige H, Horn R (2022) Environmental risk of (heavy) metal release from urns into cemetery soils. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.152952
Neckel A, Korcelski C, Kujawa HA, da Silva IS, Prezoto F, Amorin ALW, Maculan LS, Gonçalves AC, Bodah ET, Bodah BW, Dotto GL, Silva LFO (2021) Hazardous elements in the soil of urban cemeteries; constructive solutions aimed at sustainability. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.128248
Nicolaou KC, Rigol S (2018) A brief history of antibiotics and select advances in their synthesis. J Antibiot. https://doi.org/10.1038/ja.2017.62
Nunan C (2022) Ending routine farm antibiotic use in Europe. Achieving responsible farm antibiotic use through improving animal health and welfare in pig and poultry production. https://epha.org/ending-routine-farm-antibiotic-use/. Accessed 18 Feb 2023
Oliveira M, Leonardo IC, Nunes M, Silva AF, Barreto Crespo MT (2021) Environmental and pathogenic carbapenem resistant bacteria isolated from a wastewater treatment plant harbour distinct antibiotic resistance mechanism. Antibiotics. https://doi.org/10.3390/antibiotics10091118
O'Neill J (2016) Tackling drug‐resistant infections globally: final report and recommendations. The review on antimicrobial resistance. https://amr-review.org/. Accessed 30 Nov 2022
Osińska A, Korzeniewska E, Harnisz M, Felis E, Bajkacz S, Jachimowicz P, Niestępski S, Konopka I (2020) Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121221
Pacheco A, Mendes JMB, Martins T, Hassuda S, Kimmelmann AA (1991) Cemeteries—a potential risk to groundwater. Water Sci Technol. https://doi.org/10.2166/wst.1991.0341
Paíga P, Delerue-Matos C (2016) Determination of pharmaceuticals in groundwater collected in five cemeteries’ areas (Portugal). Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2016.06.090
Pawlowski AC, Johnson JW, Wright GD (2016) Evolving medicinal chemistry strategies in antibiotic discovery. Curr Opin Biotechnol. https://doi.org/10.1016/j.copbio.2016.04.006
Petrovich ML, Zilberman A, Kaplan A, Eliraz GR, Wang Y, Langenfeld K, Duhaime M, Wigginton K, Poretsky R, Avisar D, Wells GF (2020) Microbial and viral communities and their antibiotic resistance genes throughout a hospital wastewater treatment system. Front Microbiol. https://doi.org/10.3389/fmicb.2020.00153
Ribeiro da Cunha B, Fonseca LP, Calado CR (2019) Antibiotic discovery: Where have we come from, where do we go? Antibiotics. https://doi.org/10.3390/antibiotics8020045
Rodrigues L, Pacheco A (2003) Groundwater contamination from cemeteries cases of study. Environ 2010 Situat Perspect Eur Union 17:172–182
Rodriguez-Mozaz S, Chamorro S, Marti E, Huerta B, Gros M, Sànchez-Melsió A, Borrego CM, Barceló D, Balcázar JL (2015) Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. https://doi.org/10.1016/j.watres.2014.11.021
Rosenberg CR, Fang X, Allison KR (2020) Potentiating aminoglycoside antibiotics to reduce their toxic side effects. PLoS ONE. https://doi.org/10.1371/journal.pone.0237948
Rushton J (2015) Anti-microbial use in animals: how to assess the trade-offs. Zoonoses Public Health. https://doi.org/10.1111/zph.12193
Russel S (1977) Antybiotyki. PWN, Warszawa
Salmanov A, Shchehlov D, Artyomenko V, Svyrydiuk O, Maliarchuk R, Bortnik I, Mamanova M, Korniyenko S, Rud V, Gudym M, Shuba V, Loskutov O (2023) Nosocomial transmission of multi-drug-resistant organisms in Ukrainian hospitals: results of a multi-centre study (2019–2021). J Hosp Infect. https://doi.org/10.1016/j.jhin.2022.12.008
Schar D, Klein EY, Laxminarayan R, Gilbert M, Van Boeckel TP (2020) Global trends in antimicrobial use in aquaculture. Sci Rep. https://doi.org/10.1038/s41598-020-78849-3
Shi H, Hu X, Li W, Zhang J, Hu B, Lou L (2023) Soil component: a potential factor affecting the occurrence and spread of antibiotic resistance genes. Antibiotics. https://doi.org/10.3390/antibiotics12020333
Sommer MO, Dantas G, Church GM (2009) Functional characterization of the antibiotic resistance reservoir in the human microflora. Science. https://doi.org/10.1126/science.1176950
Spongberg AL, Becks PM (2000) Inorganic soil contamination from cemetery leachate. Water Air Soil Pollut. https://doi.org/10.1023/A:1005186919370
Sriram A, Kalanxhi E, Kapoor G, Craig J, Balasubramanian R, Brar S, Criscuolo N, Hamilton A, Klein E, Tseng K, Van Boeckel TP, Laxminarayan R (2021) The state of the world’s antibiotics 2021. a global analysis of antimicrobial resistance and its drivers. https://onehealthtrust.org/publications/reports/the-state-of-the-worlds-antibiotic-in-2021/. Accessed 21 Feb 2023
Główny Urząd Statystyczny (2020) Rocznik demograficzny Demographic Yearbook of Poland https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-demograficzny-2020,3,14.html. Accessed 29 Dec 2022
Stedtfeld RD, Stedtfeld TM, Waseem H, Fitschen-Brown M, Guo X, Chai B, Williams MR, Shook T, Logan A, Graham A, Chae JC, Sul WJ, VanHouten J, Cole JR, Zylstra GJ, Tiedje JM, Upham BL, Hashsham SA (2017) Isothermal assay targeting class 1 integrase gene for environmental surveillance of antibiotic resistance markers. J Environ Manag. https://doi.org/10.1016/j.jenvman.2017.04.079
Thakali O, Brooks JP, Shahin S, Sherchan SP, Haramoto E (2020) Removal of antibiotic resistance genes at two conventional wastewater treatment plants of Louisiana, USA. Water. https://doi.org/10.3390/w12061729
Thiang EL, Lee CW, Takada H, Seki K, Takei A, Suzuki S, Wang A, Bong CW (2021) Antibiotic residues from aquaculture farms and their ecological risks in Southeast Asia: a case study from Malaysia. Ecosyst Health Sust. https://doi.org/10.1080/20964129.2021.1926337
Thierauf A, Perez G, Maloy S (2009) Generalized transduction. In: Clokie MR, Kropinski AM (eds) Bacteriophages. methods in molecular biology™. Humana Press, pp 267–286. https://doi.org/10.1007/978-1-60327-164-6_23
World Health Organization (2015) Global action plan on antimicrobial resistance. https://www.who.int/publications/i/item/9789241509763. Accessed 14 Jan 2023
World Health Organization (2018) WHO report on surveillance of antibiotic consumption: 2016–2018 early implementation. https://apps.who.int/iris/handle/10665/277359. Accessed 24 Jan 2023
World Organisation for Animal Health (2016) Strategy on antimicrobial resistance and the prudent use of antimicrobials. Preserving the efficacy of antimicrobials. https://www.oie.int/en/document/en_oie-amrstrategy/. Accessed 25 Nov 2022
Xu WH, Zhang G, Zou SC, Li XD, Liu YC (2007) Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ Pollut. https://doi.org/10.1016/j.envpol.2006.05.038
Zabłotni A, Jaworski A (2014) Źródła antybiotyków w środowiskach naturalnych i ich rola biologiczna [Sources of antibiotics in natural environments and their biological role]. Postepy Hig Med Dosw. https://doi.org/10.5604/17322693.1119027
Zieliński W, Korzeniewska E, Harnisz M, Drzymała J, Felis E, Bajkacz S (2021) Wastewater treatment plants as a reservoir of integrase and antibiotic resistance genes—an epidemiological threat to workers and environment. Environ Int. https://doi.org/10.1016/j.envint.2021.106641
Żychowski J, Bryndal T (2015) Impact of cemeteries on groundwater contamination by bacteria and viruses – a review. J Water Health. https://doi.org/10.2166/wh.2014.119
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MW was involved in Conceptualization, Supervision, Writing—Reviewing and Editing. ABB contributed to Supervision, Writing—Reviewing and Editing. PT was involved in Writing—Original draft preparation, Visualization, Writing—Reviewing and Editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarnawska, P., Walczak, M. & Burkowska-But, A. Cemeteries and graveyards as potential reservoirs of antibiotic resistance genes and bacteria: a review. Environ Chem Lett 22, 297–319 (2024). https://doi.org/10.1007/s10311-023-01651-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-023-01651-w