Abstract
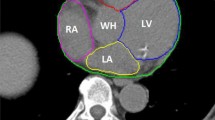
The aim of this study is to evaluate a regional deformable model based on a deep unsupervised learning model for automatic contour propagation in breast cone-beam computed tomography–guided adaptive radiation therapy. A deep unsupervised learning model was introduced to map breast’s tumor bed, clinical target volume, heart, left lung, right lung, and spinal cord from planning computed tomography to cone-beam CT. To improve the traditional image registration method’s performance, we used a regional deformable framework based on the narrow-band mapping, which can mitigate the effect of the image artifacts on the cone-beam CT. We retrospectively selected 373 anonymized cone-beam CT volumes from 111 patients with breast cancer. The cone-beam CTs are divided into three sets. 311 / 20 / 42 cone-beam CT images were used for training, validating, and testing. The manual contour was used as reference for the testing set. We compared the results between the reference and the model prediction for evaluating the performance. The mean Dice between manual reference segmentations and the model predicted segmentations for breast tumor bed, clinical target volume, heart, left lung, right lung, and spinal cord were 0.78 ± 0.09, 0.90 ± 0.03, 0.88 ± 0.04, 0.94 ± 0.03, 0.95 ± 0.02, and 0.77 ± 0.07, respectively. The results demonstrated a good agreement between the reference and the proposed contours. The proposed deep learning–based regional deformable model technique can automatically propagate contours for breast cancer adaptive radiotherapy. Deep learning in contour propagation was promising, but further investigation was warranted.





Similar content being viewed by others
Availability of Data and Material
The data are not publicly available at this time due to privacy/ethical restrictions.
Code Availability
The code that supports the findings of this study is available from the corresponding author upon reasonable request.
References
Brown LC, Mutter RW, Halyard MY: Benefits, risks, and safety of external beam radiation therapy for breast cancer. International journal of women’s health 7:449, 2015
Purdie TG, Dinniwell RE, Fyles A, Sharpe MB: Automation and intensity modulated radiation therapy for individualized high-quality tangent breast treatment plans. International Journal of Radiation Oncology* Biology* Physics 90:688–695, 2014
Yan D: Image-guided/adaptive radiotherapy: Springer, 2006
Rosu M, Dawson LA, Balter JM, McShan DL, Lawrence TS, Ten Haken RK: Alterations in normal liver doses due to organ motion. International Journal of Radiation Oncology* Biology* Physics 57:1472–1479, 2003
Stroom JC, de Boer HC, Huizenga H, Visser AG: Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. International Journal of Radiation Oncology* Biology* Physics 43:905–919, 1999
Kim LH, Vicini F, Yan D: What do recent studies on lumpectomy cavity volume change imply for breast clinical target volumes? International Journal of Radiation Oncology• Biology• Physics 72:1–3, 2008
Harris EJ, Donovan EM, Yarnold JR, Coles CE, Evans PM, Group ITM: Characterization of target volume changes during breast radiotherapy using implanted fiducial markers and portal imaging. International Journal of Radiation Oncology* Biology* Physics 73:958–966, 2009
Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA: Flat-panel cone-beam computed tomography for image-guided radiation therapy. International Journal of Radiation Oncology* Biology* Physics 53:1337–1349, 2002
Zegers CM, et al.: Three-dimensional dose evaluation in breast cancer patients to define decision criteria for adaptive radiotherapy. Acta Oncologica 56:1487-1494, 2017
Liu Y, et al.: Contour propagation using non-uniform cubic B-splines for lung tumor delineation in 4D-CT. International journal of computer assisted radiology and surgery 11:2139-2151, 2016
Yang Y, Zhou S, Shang P, Qi E, Wu S, Xie Y: Contour propagation using feature-based deformable registration for lung cancer. BioMed Research International 2013, 2013
Thor M, Petersen JB, Bentzen L, Høyer M, Muren LP: Deformable image registration for contour propagation from CT to cone-beam CT scans in radiotherapy of prostate cancer. Acta Oncologica 50:918-925, 2011
Wei D, Sun Y, Chai P, Low A, Ong SH: Myocardial segmentation of late gadolinium enhanced MR images by propagation of contours from cine MR images. Proc. International Conference on Medical Image Computing and Computer-Assisted Intervention: City
Léger J, Brion E, Javaid U, Lee J, Vleeschouwer CD, Macq B: Contour propagation in CT scans with convolutional neural networks. Proc. International Conference on Advanced Concepts for Intelligent Vision Systems: City
Eppenhof KA, et al.: Fast contour propagation for MR‐guided prostate radiotherapy using convolutional neural networks. Medical Physics 47:1238-1248, 2020
Elmahdy MS, et al.: Robust contour propagation using deep learning and image registration for online adaptive proton therapy of prostate cancer. Medical physics 46:3329-3343, 2019
Liang X, Morgan H, Nguyen D, Jiang S: Deep learning based CT-to-CBCT deformable image registration for autosegmentation in head and neck adaptive radiation therapy. arXiv preprint https://arxiv.org/abs/2102.00590, 2021
Liang X, et al.: Segmentation by Test-Time Optimization (TTO) for CBCT-based Adaptive Radiation Therapy. arXiv preprint https://arxiv.org/abs/2101.12566, 2022
Lu Y, et al.: Contour transformer network for one-shot segmentation of anatomical structures. IEEE transactions on medical imaging 40:2672-2684, 2020
Sisniega A, et al.: High-fidelity artifact correction for cone-beam CT imaging of the brain. Physics in Medicine & Biology 60:1415, 2015
Liang X, et al.: Scatter correction for a clinical cone-beam CT system using an optimized stationary beam blocker in a single scan. Medical physics 46:3165-3179, 2019
Ourselin S, Roche A, Prima S, Ayache N: Block matching: A general framework to improve robustness of rigid registration of medical images. Proc. International Conference on Medical Image Computing And Computer-Assisted Intervention: City
Hou J, Guerrero M, Chen W, D’Souza WD: Deformable planning CT to cone‐beam CT image registration in head‐and‐neck cancer. Medical physics 38:2088-2094, 2011
Zhang L, et al.: Multiple regions-of-interest analysis of setup uncertainties for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys 64:1559-1569, 2006
Schreibmann E, Xing L: Narrow band deformable registration of prostate magnetic resonance imaging, magnetic resonance spectroscopic imaging, and computed tomography studies. International Journal of Radiation Oncology* Biology* Physics 62:595–605, 2005
Chao M, Li T, Schreibmann E, Koong A, Xing L: Automated contour mapping with a regional deformable model. Int J Radiat Oncol Biol Phys 70:599-608, 2008
Chao M, Xie Y, Xing L: Auto-propagation of contours for adaptive prostate radiation therapy. Physics in Medicine & Biology 53:4533, 2008
Zhang L, et al.: Rapid histology of laryngeal squamous cell carcinoma with deep-learning based stimulated Raman scattering microscopy. Theranostics 9:2541, 2019
Si K, et al.: Fully end-to-end deep-learning-based diagnosis of pancreatic tumors. Theranostics 11:1982, 2021
Mu W, et al.: Prediction of clinically relevant Pancreatico-enteric Anastomotic Fistulas after Pancreatoduodenectomy using deep learning of Preoperative Computed Tomography. Theranostics 10:9779, 2020
Jardim-Perassi BV, et al.: Deep-learning and MR images to target hypoxic habitats with evofosfamide in preclinical models of sarcoma. Theranostics 11:5313, 2021
Jiang Y, et al.: Noninvasive Prediction of Occult Peritoneal Metastasis in Gastric Cancer Using Deep Learning. JAMA Network Open 4:e2032269-e2032269, 2021
Jiang Y, et al.: Radiographical assessment of tumour stroma and treatment outcomes using deep learning: a retrospective, multicohort study. Lancet Digital Health 3:e371-e382, 2021
Liang X, Li N, Zhang Z, Xiong J, Zhou S, Xie Y: Incorporating the Hybrid Deformable Model for Improving the Performance of Abdominal CT Segmentation via Multi-Scale Feature Fusion Network. Medical Image Analysis 73:102156, 2021
Park B, Park H, Lee SM, Seo JB, Kim N: Lung segmentation on HRCT and volumetric CT for diffuse interstitial lung disease using deep convolutional neural networks. Journal of digital imaging 32:1019-1026, 2019
Lee H, et al.: Fully automated deep learning system for bone age assessment. Journal of digital imaging 30:427-441, 2017
Hesamian MH, Jia W, He X, Kennedy P: Deep learning techniques for medical image segmentation: achievements and challenges. Journal of digital imaging 32:582-596, 2019
Schlemper J, et al.: Attention Gated Networks: Learning to Leverage Salient Regions in Medical Images. Medical Image Analysis, 2019
Balakrishnan G, Zhao A, Sabuncu MR, Guttag J, Dalca AV: VoxelMorph: a learning framework for deformable medical image registration. IEEE transactions on medical imaging 38:1788-1800, 2019
de Vos BD, Berendsen FF, Viergever MA, Sokooti H, Staring M, Isgum I: A deep learning framework for unsupervised affine and deformable image registration. Med Image Anal 52:128-143, 2019
Thirion J P. Image matching as a diffusion process: an analogy with Maxwell’s demons. Medical image analysis, 2(3): 243-260,1998
Vercauteren T, et al. Diffeomorphic demons: Efficient non-parametric image registration. NeuroImage, 45(1): S61-S72,2009
Hlavka A, et al.: Tumor bed radiotherapy in women following breast conserving surgery for breast cancer-safety margin with/without image guidance. Oncology letters 15:6009-6014, 2018
Poortmans PM, et al.: Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiotherapy and Oncology 90:80-85, 2009
Boersma LJ, et al.: Reducing interobserver variation of boost-CTV delineation in breast conserving radiation therapy using a pre-operative CT and delineation guidelines. Radiotherapy and Oncology 103:178-182, 2012
Juneja P, Harris EJ, Kirby AM, Evans PM: Adaptive breast radiation therapy using modeling of tissue mechanics: a breast tissue segmentation study. International Journal of Radiation Oncology* Biology* Physics 84:e419-e425, 2012
Elisabeth, et al.: Clinical Evaluation of Soft Tissue Organ Boundary Visualization on Cone-Beam Computed Tomographic Imaging. International Journal of Radiation Oncology*Biology*Physics, 2010
Vinod SK, Min M, Jameson MG, Holloway LC: A review of interventions to reduce inter‐observer variability in volume delineation in radiation oncology. Journal of medical imaging and radiation oncology 60:393-406, 2016
Funding
This work is partly supported by grants from the National Natural Science Foundation of China (82202954, U20A201795, U21A20480, 61871374, 12126608), Beijing Natural Science Foundation (Z210008), Young S&T Talent Training Program of Guangdong Provincial Association for S&T, China (SKXRC202224), the Chinese Academy of Sciences Special Research Assistant Grant Program, the Guangdong Provincial Hospital of Chinese Medicine Science and Technology Research Project (ZY2022YL07), and the Guangzhou Science and Technology Plan (202102010264).
Author information
Authors and Affiliations
Contributions
All of the listed authors have participated actively in the study project. Xiaokun Liang and Jingjing Dai developed the design and conduct of the study. Xuanru Zhou, Zhenhui Dai, and Xuetao Wang led the data collection. Chulong Zhang aided in the data collection. Xuanru Zhou and Na Li annotated imaging data. Lin Liu and Yuming Jiang performed data analysis and writing. Tianye Niu and Yaoqin Xie performed investigation and writing. The first draft of the manuscript was written by Xiaokun Liang and all authors commented on previous versions of the manuscript. All authors participated in and approved the final submission.
Corresponding authors
Ethics declarations
Ethics Approval
This retrospective study was approved by the ethics committee of The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (NO.BE2021-028–01).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 17583 KB)
Supplementary file2 (MP4 15251 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, X., Dai, J., Zhou, X. et al. An Unsupervised Learning-Based Regional Deformable Model for Automated Multi-Organ Contour Propagation. J Digit Imaging 36, 923–931 (2023). https://doi.org/10.1007/s10278-023-00779-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-023-00779-z