Abstract
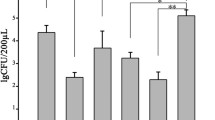
The purpose of this study was to evaluate the antibacterial efficacy of using 2.5% NaOCl, 2% chlorhexidine (CHX), Irritrol, and chitosan-coated silver nanoparticles (AgCNPs) alone or in combination with deoxyribonuclease I (DNase I) and trypsin pre-enzyme applications in dentin samples contaminated with Enterococcus faecalis (E. faecalis) by CLSM. 144 dentin blocks with confirmed E. faecalis biofilm formation were divided randomly according to the irrigation protocol (n = 12): NaOCl, CHX, Irritrol, AgCNPs, trypsin before NaOCl, CHX, Irritrol, AgCNPs, and DNase I before NaOCl, CHX, Irritrol, AgCNPs. Dentin blocks were stained with the Live/Dead BacLight Bacterial Viability Kit and viewed with CLSM after irrigation applications. The percentage of dead and viable bacteria was calculated using ImageJ software on CLSM images. At a significance level of p < 0.05, the obtained data were analyzed using one-way Anova and post-hoc Tukey tests. In comparison with NaOCl, CHX had a higher percentage of dead bacteria, both when no pre-enzyme was applied and when DNase I was applied as a pre-enzyme (p < 0.05). There was no difference in the percentage of dead bacteria between the irrigation solutions when trypsin was applied as a pre-enzyme (p > 0.05). AgCNPs showed a higher percentage of dead bacteria when trypsin was applied as a pre-enzyme compared to other irrigation solutions (p < 0.05), while the pre-enzyme application did not affect the percentage of dead bacteria in NaOCl, CHX, and Irritrol (p > 0.05). No irrigation protocol tested was able to eliminate the E. faecalis biofilm. While the application of trypsin as a pre-enzyme improved the antimicrobial effect of AgCNPs, it did not make any difference over other irrigation solutions.



Similar content being viewed by others
Data availability
This conclusion was drawn based on the results of the current study.
References
Ghorbanzadeh A, Bahador A, Sarraf P, Ayar R, Fekrazad R, Asefi S. Ex vivo comparison of antibacterial efficacy of conventional chemomechanical debridement alone and in combination with light-activated disinfection and laser irradiation against enterococcus faecalis biofilm. Photodiagnosis Photodyn Ther. 2020;29:101648.
Neelakantan P, Cheng CQ, Mohanraj R, Sriraman P, Subbarao C, Sharma S. Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er:YAG laser in vitro. Int Endod J. 2015;48(6):602–10.
Loyola-Rodriguez JP, Torres-Mendez F, Espinosa-Cristobal LF, Garcia-Cortes JO, Loyola-Leyva A, Gonzalez FJ, et al. Antimicrobial activity of endodontic sealers and medications containing chitosan and silver nanoparticles against Enterococcus faecalis. J Appl Biomater Funct Mater. 2019;17(3):2280800019851771.
Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54(2):291–312.
Bramante CM, Duque JA, Cavenago BC, Vivan RR, Bramante AS, de Andrade FB, et al. Use of a 660-nm Laser to Aid in the Healing of Necrotic Alveolar Mucosa Caused by Extruded Sodium Hypochlorite: A Case Report. J Endod. 2015;41(11):1899–902.
Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34(6):424–8.
Okino LA, Siqueira EL, Santos M, Bombana AC, Figueiredo JA. Dissolution of pulp tissue by aqueous solution of chlorhexidine digluconate and chlorhexidine digluconate gel. Int Endod J. 2004;37(1):38–41.
Essential Dental Systems. [cited; Available from: http://edsdental.com/irritrol/index.htm (30/06/20)
Kucuk M, Kermeoglu F. Efficacy of different irrigation methods on dentinal tubule penetration of Chlorhexidine, QMix and Irritrol: A confocal laser scanning microscopy study. Aust Endod J. 2019;45(2):202–8.
Samiei M, Farjami A, Dizaj SM, Lotfipour F. Nanoparticles for antimicrobial purposes in Endodontics: A systematic review of in vitro studies. Mater Sci Eng C Mater Biol Appl. 2016;58:1269–78.
Del Carpio-Perochena A, Kishen A, Shrestha A, Bramante CM. Antibacterial Properties Associated with Chitosan Nanoparticle Treatment on Root Dentin and 2 Types of Endodontic Sealers. J Endod. 2015;41(8):1353–8.
Sy K, Agossa K, Maton M, Chijcheapaza-Flores H, Martel B, Siepmann F, et al. How Adding Chlorhexidine or Metallic Nanoparticles Affects the Antimicrobial Performance of Calcium Hydroxide Paste as an Intracanal Medication: An In Vitro Study. Antibiotics (Basel). 2021;10:11.
Niazi SA, Clark D, Do T, Gilbert SC, Foschi F, Mannocci F, et al. The effectiveness of enzymic irrigation in removing a nutrient-stressed endodontic multispecies biofilm. Int Endod J. 2014;47(8):756–68.
Jakubovics NS, Shields RC, Rajarajan N, Burgess JG. Life after death: the critical role of extracellular DNA in microbial biofilms. Lett Appl Microbiol. 2013;57(6):467–75.
Rodrigues CT, de Andrade FB, de Vasconcelos L, Midena RZ, Pereira TC, Kuga MC, et al. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int Endod J. 2018;51(8):901–11.
Wang N, Ji Y, Zhu Y, Wu X, Mei L, Zhang H, et al. Antibacterial effect of chitosan and its derivative on Enterococcus faecalis associated with endodontic infection. Exp Ther Med. 2020;19(6):3805–13.
Bukhari S, Kim D, Liu Y, Karabucak B, Koo H. Novel Endodontic Disinfection Approach Using Catalytic Nanoparticles. J Endod. 2018;44(5):806–12.
Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol. 1971;32(2):271–5.
Ruiz-Linares M, Aguado-Perez B, Baca P, Arias-Moliz MT, Ferrer-Luque CM. Efficacy of antimicrobial solutions against polymicrobial root canal biofilm. Int Endod J. 2017;50(1):77–83.
Swimberghe RCD, Coenye T, De Moor RJG, Meire MA. Biofilm model systems for root canal disinfection: a literature review. Int Endod J. 2019;52(5):604–28.
Ganesh A, Nagendrababu V, John A, Deivanayagam K. The Effect of Addition of an EPS Degrading Enzyme with and without Detergent to 2% Chlorhexidine on Disruption of Enterococcus faecalis Biofilm: A Confocal Laser Scanning Microscopic Study. J Clin Diagn Res. 2015;9(11):ZC61-65.
Li W, Liu H, Xu Q. Extracellular dextran and DNA affect the formation of Enterococcus faecalis biofilms and their susceptibility to 2% chlorhexidine. J Endod. 2012;38(7):894–8.
Niazi SA, Al-Ali WM, Patel S, Foschi F, Mannocci F. Synergistic effect of 2% chlorhexidine combined with proteolytic enzymes on biofilm disruption and killing. Int Endod J. 2015;48(12):1157–67.
Torelli R, Cacaci M, Papi M, Paroni Sterbini F, Martini C, Posteraro B, et al. Different effects of matrix degrading enzymes towards biofilms formed by E. faecalis and E. faecium clinical isolates. Colloids Surf B Biointerfaces. 2017;158:349–55.
Bin Ahmad M, Lim JJ, Shameli K, Ibrahim NA, Tay MY. Synthesis of silver nanoparticles in chitosan, gelatin and chitosan/gelatin bionanocomposites by a chemical reducing agent and their characterization. Molecules. 2011;16(9):7237–48.
Alsubait S, Albader S, Alajlan N, Alkhunaini N, Niazy A, Almahdy A. Comparison of the antibacterial activity of calcium silicate- and epoxy resin-based endodontic sealers against Enterococcus faecalis biofilms: a confocal laser-scanning microscopy analysis. Odontology. 2019;107(4):513–20.
Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32(2):93–8.
Seneviratne CJ, Yip JW, Chang JW, Zhang CF, Samaranayake LP. Effect of culture media and nutrients on biofilm growth kinetics of laboratory and clinical strains of Enterococcus faecalis. Arch Oral Biol. 2013;58(10):1327–34.
Stojicic S, Shen Y, Haapasalo M. Effect of the source of biofilm bacteria, level of biofilm maturation, and type of disinfecting agent on the susceptibility of biofilm bacteria to antibacterial agents. J Endod. 2013;39(4):473–7.
Siqueira JF Jr, Antunes HS, Rocas IN, Rachid CT, Alves FR. Microbiome in the Apical Root Canal System of Teeth with Post-Treatment Apical Periodontitis. PLoS ONE. 2016;11(9): e0162887.
Swimberghe RCD, Crabbe A, De Moor RJG, Coenye T, Meire MA. Model system parameters influence the sodium hypochlorite susceptibility of endodontic biofilms. Int Endod J. 2021;54(9):1557–70.
Thomas VC, Hiromasa Y, Harms N, Thurlow L, Tomich J, Hancock LE. A fratricidal mechanism is responsible for eDNA release and contributes to biofilm development of Enterococcus faecalis. Mol Microbiol. 2009;72(4):1022–36.
Guiton PS, Hung CS, Kline KA, Roth R, Kau AL, Hayes E, et al. Contribution of autolysin and Sortase a during Enterococcus faecalis DNA-dependent biofilm development. Infect Immun. 2009;77(9):3626–38.
Tetz VV, Tetz GV. Effect of extracellular DNA destruction by DNase I on characteristics of forming biofilms. DNA Cell Biol. 2010;29(8):399–405.
Jacinto RC, Gomes BP, Shah HN, Ferraz CC, Zaia AA, Souza-Filho FJ. Quantification of endotoxins in necrotic root canals from symptomatic and asymptomatic teeth. J Med Microbiol. 2005;54(Pt 8):777–83.
Zapata RO, Bramante CM, de Moraes IG, Bernardineli N, Gasparoto TH, Graeff MS, et al. Confocal laser scanning microscopy is appropriate to detect viability of Enterococcus faecalis in infected dentin. J Endod. 2008;34(10):1198–201.
Amos WB, White JG. How the Confocal Laser Scanning Microscope entered Biological Research. Biol Cell. 2003;95(6):335–42.
Kishen A, Shrestha A, Del Carpio-Perochena A. Validation of Biofilm Assays to Assess Antibiofilm Efficacy in Instrumented Root Canals after Syringe Irrigation and Sonic Agitation. J Endod. 2018;44(2):292–8.
Guerreiro-Tanomaru JM, Nascimento CA, Faria-Junior NB, Graeff MS, Watanabe E, Tanomaru-Filho M. Antibiofilm activity of irrigating solutions associated with cetrimide. Confocal laser scanning microscopy. Int Endod J. 2014;47(11):1058–63.
Netuschil L, Auschill TM, Sculean A, Arweiler NB. Confusion over live/dead stainings for the detection of vital microorganisms in oral biofilms–which stain is suitable? BMC Oral Health. 2014;14:2.
Menezes MM, Valera MC, Jorge AO, Koga-Ito CY, Camargo CH, Mancini MN. In vitro evaluation of the effectiveness of irrigants and intracanal medicaments on microorganisms within root canals. Int Endod J. 2004;37(5):311–9.
Dametto FR, Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, de Souza-Filho FJ. In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as an endodontic irrigant against Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(6):768–72.
Hope CK, Garton SG, Wang Q, Burnside G, Farrelly PJ. A direct comparison between extracted tooth and filter-membrane biofilm models of endodontic irrigation using Enterococcus faecalis. Arch Microbiol. 2010;192(9):775–81.
Ma J, Wang Z, Shen Y, Haapasalo M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod. 2011;37(10):1380–5.
Saricam E, Kucuk M, Akyol M. Evaluation of EDTA, QMix, and Irritrol solutions activated with Er, Cr:YSGG and diode lasers on the push-out bond strength of filling material. Microsc Res Tech. 2021;84(4):584–91.
Ballal NV, Narkedamalli R, Gandhi P, Arias-Moliz MT, Baca P, Das S, et al. Biological and chemical properties of 2-in-1 calcium-chelating and antibacterial root canal irrigants. J Dent. 2023;134: 104526.
Moghadas L, Shahmoradi M, Narimani T. Antimicrobial activity of a new nanobased endodontic irrigation solution: In vitro study. Dental Hypotheses. 2012;3(4):142.
Jaiswal N, Sinha DJ, Singh UP, Singh K, Jandial UA, Goel S. Evaluation of antibacterial efficacy of Chitosan, Chlorhexidine, Propolis and Sodium hypochlorite on Enterococcus faecalis biofilm : An in vitro study. J Clin Exp Dent. 2017;9(9):e1066–74.
Afkhami F, Akbari S, Chiniforush N. Entrococcus faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-activated Nanoparticles: An In Vitro Study. J Endod. 2017;43(2):279–82.
Yu MK, Kim MA, Rosa V, Hwang YC, Del Fabbro M, Sohn WJ, et al. Role of extracellular DNA in Enterococcus faecalis biofilm formation and its susceptibility to sodium hypochlorite. J Appl Oral Sci. 2019;27: e20180699.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doğan Çankaya, T., Uğur Aydın, Z. & Erdönmez, D. The effect of the enzymes trypsin and DNase I on the antimicrobial efficiency of root canal irrigation solutions. Odontology (2024). https://doi.org/10.1007/s10266-023-00894-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10266-023-00894-x