Abstract
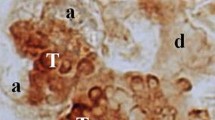
In the present study, expression and localization of PLD2 were examined in mouse submandibular glands in situ in comparison with those of PLD1 previously reported by the present authors. In immunoblots, PLD2-expression was high at postnatal 0 week (P0W) and P2W, and at P4W decreased considerably with the decrease of the male gland more marked, and at P8W it was undetectable in the male gland, but remained faint in the female. In the male gland at P8W after castration at P6W, PLD2-expression reappeared faintly. In the female glands with daily injections of testosterone started at P6W, at P8W the expression was undetectable. In immunohistochemistry, PLD2 was localized throughout immature duct epithelia without distinct regional differences in its intensity at P0W and P2W. PLD2-localization was weak and diffuse in granular convoluted tubules at P4W and negligible there at P8W in the female gland, while it was at negligible levels in the tubules at P4W and at undetectable levels there at P8W in the male gland. The expression changes detected in immunoblots after castration or testosterone injection were duplicated in granular convoluted tubules in immunohistochemistry. The present findings suggest that PLD2 is dominantly involved in proliferation/differentiation/apoptosis of most immature ductal cells at early postnatal and at least perinatal stages, different from PLD1. This also indicates that the female-dominant sexual dimorphism of PLD2-expression occurs in synchrony of differentiation of granular convoluted tubules at puberty and at young adulthood, similar to PLD1 though at much lower levels.




Similar content being viewed by others
Data availability
All data of this study are available from the corresponding author upon reasonable request.
References
Banno Y, Fujita H, Ono Y, Nakashima S, Ito Y, Kuzumaki N, Nozawa Y. Differential phospholipase D activation by bradykinin and sphingosine 1-phosphate in NIH 3T3 fibroblasts overexpressing gelsolin. J Biol Chem. 1999;274(39):27385–91.
Liscovitch M, Czarny M, Fiucci G, Tang X. Phospholipase D: molecular and cell biology of a novel gene family. Biochem J. 2000;345(Pt-3):401–15.
Nozawa Y. Roles of phospholipase D in apoptosis and pro-survival. Biochim Biophys Acta. 2002;1585(2–3):77–86.
Brown HA, Henage LG, Preininger AM, Xiang Y, Exton JH. Biochemical analysis of phospholipase D. Methods Enzymol. 2007;434:49–87.
Nelson RK, Frohman MA. Physiological and pathophysiological roles for phospholipase D. J Lipid Res. 2015;56(12):2229–37.
Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194(2):77–89.
Wang Y, Chen X, Lian L, Tang T, Stalker TJ, Sasaki T, Kanaho Y, Brass LF, Choi JK, Hartwig JH, Abrams CS. Loss of PIP5KIbeta demonstrates that PIP5KI isoform-specific PIP2 synthesis is required for IP3 formation. Proc Natl Acad Sci U S A. 2008;105(37):14064–9.
Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7(3):191–201.
Du G, Altshuller YM, Kim Y, Han JM, Ryu SH, Morris AJ, Frohman MA. Dual requirement for rho and protein kinase C in direct activation of phospholipase D1 through G protein-coupled receptor signaling. Mol Biol Cell. 2000;11(12):4359–68.
Freyberg Z, Sweeney D, Siddhanta A, Bourgoin S, Frohman M, Shields D. Intracellular localization of phospholipase D1 in mammalian cells. Mol Biol Cell. 2001;12(4):943–55.
Du G, Huang P, Liang BT, Frohman MA. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell. 2004;15(3):1024–30.
Peng X, Frohman MA. Mammalian phospholipase D physiological and pathological roles. Acta Physiol. 2012;204(2):219–26.
Freyberg Z, Bourgoin S, Shields D. Phospholipase D2 is localized to the rims of the Golgi apparatus in mammalian cells. Mol Biol Cell. 2002;13(11):3930–42.
Sarri E, Pardo R, Fensome-Green A, Cockcroft S. Endogenous phospholipase D2 localizes to the plasma membrane of RBL-2H3 mast cells and can be distinguished from ADP ribosylation factor-stimulated phospholipase D1 activity by its specific sensitivity to oleic acid. Biochem J. 2003;369(Pt 2):319–29.
Koch T, Wu DF, Yang LQ, Brandenburg LO, Höllt V. Role of phospholipase D2 in the agonist-induced and constitutive endocytosis of G-protein coupled receptors. J Neurochem. 2006;97(2):365–72.
Khrongyut S, Polsan Y, Sakaew W, Sawatpanich T, Banno Y, Nozawa Y, Kondo H, Hipkaeo W. Expression of endogenous phospholipase D1, localized in mouse submandibular gland, is greater in females and is suppressed by testosterone. J Anat. 2019;235(6):1125–36.
Zhao Y, Ehara H, Akao Y, Shamoto M, Nakagawa Y, Banno Y, Deguchi T, Ohishi N, Yagi K, Nozawa Y. Increased activity and intranuclear expression of phospholipase D2 in human renal cancer. Biochem Biophys Res Commun. 2000;278(1):140–3.
Nakamura N, Banno Y, Tamiya-Koizumi K. Arf1-dependent PLD1 is localized to oleic acid-induced lipid droplets in NIH3T3 cells. Biochem Biophys Res Commun. 2005;335(1):117–23.
Gresik EW. Postnatal developmental changes in submandibular glands of rats and mice. J Histochem Cytochem. 1980;28(8):860–70.
Gresik EW. The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microsc Res Tech. 1994;27(1):1–24.
Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74(7):349–64.
Bollag WB, Xie D, Zheng X, Zhong X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: production of a phosphatidylglycerol signaling lipid. J Invest Dermatol. 2007;127(12):2823–31.
Banno Y, Nemoto S, Murakami M, et al. Depolarization-induced differentiation of PC12 cells is mediated by phospholipase D2 through the transcription factor CREB pathway. J Neurochem. 2008;104(5):1372–86.
Caramia F. Ultrastructure of mouse submaxillary gland. I Sexual differences J Ultrastruct Res. 1966;16(5):505–23.
Chrétien M. Action of testosterone on the differentiation and secretory activity of a target organ: the submaxillary gland of the mouse. Int Rev Cytol. 1977;50:333–96.
Pinkstaff CA. The cytology of salivary glands. Int Rev Cytol. 1980;63:141–261.
Hipkaeo W, Wakayama T, Yamamoto M, Iseki S. Expression and localization of the transcription factor JunD in the duct system of mouse submandibular gland. J Histochem Cytochem. 2004;52(4):479–90.
Yamamoto M, Nakata H, Kumchantuek T, Adhapanyawanich K, Iseki S. Distinct hormonal regulation of two types of sexual dimorphism in submandibular gland of mice. Cell Tissue Res. 2018;371(2):261–72.
Acknowledgements
The authors would like to thank Mr. Hipkaeo D., Electron Microscopy Unit, Department of Anatomy, Faculty of Medicine, Khon Kaen University, Thailand, for his technical support. We would like to acknowledge Prof. James Arthur Will, for editing the MS via Publication Clinic KKU, Thailand.
Funding
The authors are grateful for research grants from the Faculty of Medicine, Khon Kaen University (No. IN65201 to SC).
Author information
Authors and Affiliations
Contributions
HK: conceived and designed the research and edited the manuscript. SC and WH: performed Western blot and immunohistochemical analyses and wrote the core of manuscript. SK, SP, AW and AP: analyzed the data. YB and YN: synthesized PLD2 antibody and characterized it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khrongyut, S., Pakkarato, S., Watthanakitphibun, A. et al. Expression with early postnatal peak and female-dominant sexual dimorphism of phospholipase D (PLD) 2 in submandibular gland ducts in situ of mice. Odontology 111, 565–572 (2023). https://doi.org/10.1007/s10266-022-00765-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-022-00765-x