Abstract
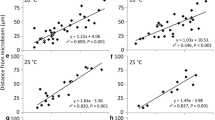
Chloroplast photorelocation movement is important for plants to perform efficient photosynthesis. Phototropins were identified as blue-light receptors for chloroplast movement in Arabidopsis thaliana and in the fern Adiantum capillus-veneris, whereas neochrome functions as a dual red/blue light receptor in the latter. However, the signal transduction pathways involved in chloroplast movement remain to be clarified. To investigate the kinetic properties of signalling from these photoreceptors to the chloroplasts, we deduced the speed of signal transfer using Adiantum capillus-veneris gametophytes. When a region of dark-adapted gametophyte cells was subjected to microbeam irradiation, chloroplasts moved towards the irradiated area even in subsequent darkness. We therefore recorded the movement and calculated the speeds of signal transfer by time-lapse imaging. Movement speeds under red or blue light were similar, e.g., about 1.0 μm min−1 in prothallial cells. However, speeds varied according to cell polarity in protonemal cells. The speed of signal transfer from the protonemal apex to the base was approximately 0.7 μm min−1, but roughly 2.3 μm min−1 in the opposite direction. The speed of signal transfer in Arabidopsis thaliana mesophyll cells was approximately 0.8 μm min−1 by comparison. Surprisingly, chloroplasts located farthest away from the microbeam were found to move faster than those in close proximity to the site of irradiation both in Adiantum capillus-veneris and A. thaliana.







Similar content being viewed by others
References
Ahmad M, Cashmore AR (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:162–166
Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282:1698–1701
Haupt W (1999) Chloroplast movement: from phenomenology to molecular biology. Prog Bot 60:3–36
Hayama R, Coupland G (2003) Shedding light on the circadian clock and the photoperiodic control of flowering. Curr Opin Plant Biol 6:13–19
Iino M (2001) Phototropism in higher plants in photomovement. In: Häder D, Lebert M (eds) ESP comprehensive series in photosciences, vol 1. Elsevier, Amsterdam, pp 659–811
Ishikawa R, Tamaki S, Yokoi S, Inagaki N, Shinomura T, Takano M, Shimamoto K (2005) Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell 17:3326–3336
Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410:952–954
Kadota A, Wada M (1992) Photoorientation of chloroplasts in protonemal cells of the fern Adiantum as analyzed by use of a video-tracking system. Bot Mag Tokyo 105:265–279
Kadota A, Wada M (1999) Red light-aphototropic (rap) mutants lack red light-induced chloroplast relocation movement in the fern Adiantum capillus-veneris. Plant Cell Physiol 40:238–247
Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoji K, Ichikawa S, Kagawa T, Nakano A, Wada M (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci USA 106:13106–13111
Kagawa T, Wada M (1996) Phytochrome and blue light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum as analyzed by microbeam irradiation. Planta 198:488–493
Kagawa T, Wada M (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol 41:84–93
Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homologue controlling the chloroplast high-light avoidance response. Science 291:2138–2141
Kagawa T, Kasahara M, Abe T, Yoshida S, Wada M (2004) Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol 45:416–426
Kanegae T, Wada M (2006) Photomorphogenesis in ferns. In: Schaefer E, Nagy F (eds) Photomorphogenesis in plants and bacteria, 3rd edn. Springer, Dordrecht, pp 515–536
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420:829–832
Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada M (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421:287–290
Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414:656–660
Kubota HY, Yoshimoto Y, Yoneda M, Hiramoto Y (1987) Free calcium wave upon activation in Xenopus eggs. Dev Biol 119:129–136
Linke WA, Bartoo ML, Pollack GH (1993) Spontaneous sarcomeric oscillations at intermediate activation levels in single isolated cardiac myofibrils. Circ Res 73:724–734
Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue-light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98:6969–6974
Sato Y, Wada M, Kadota A (2001) Choice of tracks, microtubules and/or actin filaments for chloroplast photo-movement is differentially controlled by phytochrome and a blue light receptor. J Cell Sci 114:269–279
Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M (1996) Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA 93:8129–8133
Suetsugu N, Wada M (2007) Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biol Chem 388:927–935
Sugai M, Furuya M (1967) Photomorphogenesis in Pteris vittata. I. Phytochrome-mediated spore germination and blue light interaction. Plant Cell Physiol 8:737–748
Tlalka M, Fricker M (1999) The role of calcium in blue-light-dependent chloroplast movement in Lemna trisulca L. Plant J 20:461–473
Tsuboi H, Suetsugu N, Wada M (2006) Negative phototropic response of rhizoid cells in the fern Adiantum capillus-veneris. J Plant Res 119:505–512
Tsuboi H, Suetsugu N, Kawai-Toyooka H, Wada M (2007) Phototropins and neochrome1 mediate nuclear movement in the fern Adiantum capillus-veneris. Plant Cell Physiol 48:892–896
Tsuboi H, Yamashita H, Wada M (2009) Chloroplasts do not have a polarity for light-induced accumulation movement. J Plant Res 122:131–140
Tucker EB, Lee M, Alli S, Sookhdeo V, Wada M, Imaizumi T, Kasahara M, Hepler PK (2005) UV-A induces two calcium waves in Physcomitrella patens. Plant Cell Physiol 46:1226–1236
Vicker MG (1994) The regulation of chemotaxis and chemokinesis in Dictyostellium amoebae by temporal signals and spatial gradients of cyclic AMP. J Cell Sci 107:659–667
Wada M (2007) The fern as a model system to study photomorphogenesis. J Plant Res 120:3–16
Wada M, Mineyuki Y, Furuya M (1982) Changes in the rate of organelle movement during progression of the cell cycle in Adiantum protonemata. Protoplasma 113:132–136
Wada M, Kadota A, Furuya M (1983) Intracellular localization and dichroic orientation of phytochrome in plasma membrane and/or ectoplasm of a centrifuged protonema of fern Adiantum capillus-veneris. Plant Cell Physiol 24:1441–1447
Wada M, Kagawa T, Sato Y (2003) Chloroplast movement. Annu Rev Plant Biol 54:455–468
Yatsuhashi H, Wada M (1990) High-fluence rate response in the light-oriented chloroplast movement in Adiantum protonemata. Plant Sci 68:87–94
Acknowledgments
We thank Dr. John Christie, University of Glasgow, for his critical reading and editing of this manuscript. This work was partly supported by the Japanese Ministry of Education, Sports, Science, and Technology (MEXT 13139203, 17084006 to M.W.), the Japan Society of Promotion of Science (JSPS 13304061, 16107002, 20227001 to M.W.), and a Research Fellowship for Young Scientists (to H.T.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuboi, H., Wada, M. Speed of signal transfer in the chloroplast accumulation response. J Plant Res 123, 381–390 (2010). https://doi.org/10.1007/s10265-009-0284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-009-0284-y