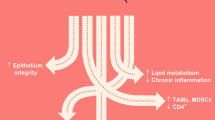
Abstract
Chemokines were originally defined as cytokines that affect the movement of immune cells. In recent years, due to the increasing importance of immune cells in the tumor microenvironment (TME), the role of chemokines has changed from a single "chemotactic agent" to a key factor that can regulate TME and affect the tumor phenotype. CXCL6, also known as granulocyte chemoattractant protein-2 (GCP-2), can recruit neutrophils to complete non-specific immunity in the process of inflammation. Cancer-related genes and interleukin family can promote the abnormal secretion of CXCL6, which promotes tumor growth, metastasis, epithelial mesenchymal transformation (EMT) and angiogenesis in the TME. CXCL6 also has a role in promoting fibrosis and tissue damage repair. In this review, we focus on the regulatory network affecting CXCL6 expression, its role in the progress of inflammation and how it affects tumorigenesis and progression based on the TME, in an attempt to provide a potential target for the treatment of diseases such as inflammation and cancer.



Similar content being viewed by others
Data availability and materials
Not applicable.
Change history
26 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10238-023-01209-8
Abbreviations
- TME:
-
Tumor microenvironment
- GCP-2:
-
Granulocyte chemoattractant protein-2
- EMT:
-
Epithelial mesenchymal transformation
- HCC:
-
Hepatocellular carcinoma
- PTX:
-
Pertussis toxin
- ACKR:
-
Atypical chemokine receptors
- ELR:
-
Glutamic acid–leucine–arginine
- RA:
-
Rheumatoid arthritis
- NETs:
-
Neutrophil extracellular traps
- FLSs:
-
Fibroblast-like synoviocytes
- FLS-OA:
-
Osteoarthritic fibroblast-like synoviocytes
- LPS:
-
Lipopolysaccharide
- TNF-α:
-
Tumor necrosis factor-α
- CAP1:
-
Cyclase-associated protein 1
- HDAC:
-
Histone deacetylase
- IL-1β:
-
Interleukin-1β
- DN:
-
Diabetes nephropathy
- AKI:
-
Acute renal injury
- ARPCs:
-
Adult renal progenitor cells
- PPARα:
-
Peroxisome proliferator-activated receptor α
- HRSV:
-
Human respiratory syncytial virus
- PITX2:
-
Paired-like homeodomain transcription factor 2
- GC:
-
Glucocorticoid
- IBD:
-
Inflammatory bowel disease
- AD:
-
Atopic dermatitis
- T1R:
-
Type 1 response
- BPH:
-
Benign prostatic hyperplasia
- TSPAN12:
-
Tetraspanin 12
- RNF152:
-
Ring finger protein 152
- PAI-1:
-
Plasminogen activator inhibitor-1
- CLCF1:
-
Cardiotrophin-like cytokine factor 1
- CAF:
-
Cancer-associated fibroblasts
- NSCLC:
-
Non-small cell lung cancer
- Th17:
-
T cell helper 17
- AML:
-
Acute myeloid leukemia
- VEGF:
-
Vascular endothelial growth factor
- TAM:
-
Tumor-associated macrophages
- ESCC:
-
Esophageal squamous cell carcinoma
- PD-L1:
-
Programmed cell death ligand 1
- HIF-α:
-
Hypoxia-inducible factor-1α
- TANs:
-
Tumor-associated neutrophils
- BRD7:
-
Bromodomain-containing protein 7
- SSc:
-
Systemic sclerosis
- HUVECs:
-
Human umbilical vein endothelial cells
- Fli1:
-
Friend leukemia virus integration 1
- HGF:
-
Hepatocyte growth factor
References
Vandercappellen J, Noppen S, Verbeke H, et al. Stimulation of angiostatic platelet factor-4 variant (CXCL4L1/PF-4var) versus inhibition of angiogenic granulocyte chemotactic protein-2 (CXCL6/GCP-2) in normal and tumoral mesenchymal cells. J Leukoc Biol. 2007;826:1519–30.
Matter MS, Marquardt JU, Andersen JB, et al. Oncogenic driver genes and the inflammatory microenvironment dictate liver tumor phenotype. Hepatology. 2016;636:1888–99.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;4206917:860–7.
Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;4577225:36–7.
Wuyts A, Struyf S, Gijsbers K, et al. The CXC chemokine GCP-2/CXCL6 is predominantly induced in mesenchymal cells by interleukin-1beta and is down-regulated by interferon-gamma: comparison with interleukin-8/CXCL8. Lab Invest. 2003;831:23–34.
Norlander AE, Saleh MA, Madhur MS. CXCL16: a chemokine-causing chronic kidney disease. Hypertension. 2013;626:1008–10.
B. J Rollins Chemokines Blood. 1997;903:909–28.
Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;211:1174–8.
Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702.
Bonecchi R, Graham GJ. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front Immunol. 2016;7:224.
Mu L, Hu S, Li G, et al. Characterization of the prognostic values of CXCL family in Epstein–Barr virus associated gastric cancer. Oxid Med Cell Longev. 2022;2022:2218140.
Proost P, De Wolf-Peeters C, Conings R, Opdenakker G, Billiau A, Van Damme J. Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO, IP-10, and IL-8. J Immunol. 1993;1503:1000–10.
Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;3387:436–45.
Verbeke H, Struyf S, Berghmans N, et al. Isotypic neutralizing antibodies against mouse GCP-2/CXCL6 inhibit melanoma growth and metastasis. Cancer Lett. 2011;3021:54–62.
Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S. How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways. Cell Signal. 2019;54:69–80.
Madalli S, Beyrau M, Whiteford J, et al. Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol Sex Differ. 2015;6:27.
Li MY, Zhao Y, Luo YB, Li YH, Liu Y. The effect and mechanism of transient receptor potential M(2) in antigen-induced arthritis mice. Zhonghua Nei Ke Za Zhi. 2019;5812:911–4.
Chwastek J, Kędziora M, Borczyk M, Korostyński M, Starowicz K. Inflammation-driven secretion potential is upregulated in osteoarthritic fibroblast-like synoviocytes. Int J Mol Sci. 2022;23(19):11817.
Sato H, Muraoka S, Kusunoki N, et al. Resistin upregulates chemokine production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res Ther. 2017;191:263.
Choo QY, Ho PC, Tanaka Y, Lin HS. The histone deacetylase inhibitors MS-275 and SAHA suppress the p38 mitogen-activated protein kinase signaling pathway and chemotaxis in rheumatoid arthritic synovial fibroblastic E11 cells. Molecules. 2013;1811:14085–95.
Angiolilli C, Kabala PA, Grabiec AM, et al. Control of cytokine mRNA degradation by the histone deacetylase inhibitor ITF2357 in rheumatoid arthritis fibroblast-like synoviocytes: beyond transcriptional regulation. Arthritis Res Ther. 2018;201:148.
Sun MY, Wang SJ, Li XQ, et al. CXCL6 promotes renal interstitial fibrosis in diabetic nephropathy by activating JAK/STAT3 signaling pathway. Front Pharmacol. 2019;10:224.
Wang SZ, Zhang YL, Shi HB. Potential repressive impact of microRNA-20a on renal tubular damage in diabetic kidney disease by targeting C-X-C motif chemokine ligand 6. Arch Med Res. 2021;521:58–68.
Sallustio F, Stasi A, Curci C, et al. Renal progenitor cells revert LPS-induced endothelial-to-mesenchymal transition by secreting CXCL6, SAA4, and BPIFA2 antiseptic peptides. Faseb j. 2019;3310:10753–66.
Jiang Y, Xi Y, Li Y, et al. Ethanol promoting the upregulation of C-X-C Motif Chemokine Ligand 1 (CXCL1) and C-X-C Motif Chemokine Ligand 6 (CXCL6) in models of early alcoholic liver disease. Bioengineered. 2022;133:4688–701.
Janssen AW, Betzel B, Stoopen G, et al. The impact of PPARα activation on whole genome gene expression in human precision cut liver slices. BMC Genomics. 2015;16:760.
Wang H, Chavali S, Mobini R, et al. A pathway-based approach to find novel markers of local glucocorticoid treatment in intermittent allergic rhinitis. Allergy. 2011;661:132–40.
Bao L, Shi VY, Chan LS. IL-4 up-regulates epidermal chemotactic, angiogenic, and pro-inflammatory genes and down-regulates antimicrobial genes in vivo and in vitro: relevant in the pathogenesis of atopic dermatitis. Cytokine. 2013;612:419–25.
Girolomoni G, Mrowietz U, Paul C. Psoriasis: rationale for targeting interleukin-17. Br J Dermatol. 2012;1674:717–24.
Fang S, Xu X, Zhong L, et al. Bioinformatics-based study to identify immune infiltration and inflammatory-related hub genes as biomarkers for the treatment of rheumatoid arthritis. Immunogenetics. 2021;736:435–48.
Weissmann G, Korchak H. Rheumatoid arthritis. The role of neutrophil activation. Inflammation. 1984;8(Suppl):S3-14.
Demoruelle MK, Harrall KK, Ho L, et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol. 2017;696:1165–75.
Chen W, Wang Q, Ke Y, Lin J. Neutrophil function in an inflammatory milieu of rheumatoid arthritis. J Immunol Res. 2018;2018:8549329.
Appelgren D, Enocsson H, Skogman BH, et al. Neutrophil extracellular traps (NETs) in the cerebrospinal fluid samples from children and adults with central nervous system Infections. Cells. 2019;9(1):43.
Jovic S, Linge HM, Shikhagaie MM, et al. The neutrophil-recruiting chemokine GCP-2/CXCL6 is expressed in cystic fibrosis airways and retains its functional properties after binding to extracellular DNA. Mucosal Immunol. 2016;91:112–23.
Linge HM, Collin M, Nordenfelt P, Mörgelin M, Malmsten M, Egesten A. The human CXC chemokine granulocyte chemotactic protein 2 (GCP-2)/CXCL6 possesses membrane-disrupting properties and is antibacterial. Antimicrob Agents Chemother. 2008;527:2599–607.
Hulander E, Bärebring L, Turesson Wadell A, et al. Proposed anti-inflammatory diet reduces inflammation in compliant, weight-stable patients with rheumatoid arthritis in a randomized controlled crossover trial. J Nutr. 2021;15112:3856–64.
Petra H, Eva H, Irena B, Petra H, Ondřej V. Molecular profiling of acute and chronic rejections of renal allografts. Clin Dev Immunol. 2013;2013:509259.
Shen YL, Jiang YP, Li XQ, et al. ErHuang formula improves renal fibrosis in diabetic nephropathy rats by inhibiting CXCL6/JAK/STAT3 signaling pathway. Front Pharmacol. 2019;10:1596.
Krupickova L, Fialova M, Novotny M, et al. Chemokine profiles are affected in serum of patients with acute rejection of kidney allograft. Mediators Inflamm. 2021;2021:5513690.
Zeng M, Liu J, Yang W, et al. Multiple-microarray analysis for identification of hub genes involved in tubulointerstial injury in diabetic nephropathy. J Cell Physiol. 2019;234:16447–62.
Wang X, Li J, Wang Z, Deng A. Wound exudate CXCL6: a potential biomarker for wound healing of diabetic foot ulcers. Biomark Med. 2019;133:167–74.
Sehrawat TS, Liu M, Shah VH. The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol Hepatol. 2020;55:494–506.
Liu M, Cao S, He L, et al. Super enhancer regulation of cytokine-induced chemokine production in alcoholic hepatitis. Nat Commun. 2021;121:4560.
Dominguez M, Miquel R, Colmenero J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;1365:1639–50.
Chen M, Xing J, Pan D, Peng X, Gao P. Chinese herbal medicine mixture 919 syrup alleviates nonalcoholic fatty liver disease in rats by inhibiting the NF-κB pathway. Biomed Pharmacother. 2020;128:110286.
Xu MY, Qu Y, Li Z, Li F, Xiao CY, Lu LG. A 6 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis B. Front Biosci (Landmark Ed). 2016;213:479–86.
Aregay A, Engel B, Port K, et al. Distinct immune imprints of post-liver transplantation hepatitis C persist despite viral clearance. Liver Transpl. 2021;276:887–99.
Sachse F, Ahlers F, Stoll W, Rudack C. Neutrophil chemokines in epithelial inflammatory processes of human tonsils. Clin Exp Immunol. 2005;1402:293–300.
Jang Y, Seo SH. Gene expression pattern differences in primary human pulmonary epithelial cells infected with MERS-CoV or SARS-CoV-2. Arch Virol. 2020;16510:2205–11.
Touzelet O, Broadbent L, Armstrong SD, et al. The secretome profiling of a pediatric airway epithelium infected with hRSV identified aberrant apical/basolateral trafficking and novel immune modulating (CXCL6, CXCL16, CSF3) and antiviral (CEACAM1) proteins. Mol Cell Proteomics. 2020;195:793–807.
Moazzeni H, Akbari MT, Yazdani S, Elahi E. Expression of CXCL6 and BBS5 that may be glaucoma relevant genes is regulated by PITX2. Gene. 2016;5931:76–83.
Chen C, Shi L, Li Y, Wang X, Yang S. Disease-specific dynamic biomarkers selected by integrating inflammatory mediators with clinical informatics in ARDS patients with severe pneumonia. Cell Biol Toxicol. 2016;323:169–84.
Xu L, Duda DG, di Tomaso E, et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;6920:7905–10.
Holmgren K, Jonsson P, Lundin C, et al. Preoperative biomarkers related to inflammation may identify high-risk anastomoses in colorectal cancer surgery: explorative study. BJS Open. 2022. https://doi.org/10.1093/bjsopen/zrac072.
Gijsbers K, Van Assche G, Joossens S, et al. CXCR1-binding chemokines in inflammatory bowel diseases: down-regulated IL-8/CXCL8 production by leukocytes in Crohn’s disease and selective GCP-2/CXCL6 expression in inflamed intestinal tissue. Eur J Immunol. 2004;347:1992–2000.
Boshagh MA, Foroutan P, Moloudi MR, et al. ELR positive CXCL chemokines are highly expressed in an animal model of ulcerative colitis. J Inflamm Res. 2019;12:167–74.
Khaiboullina SF, Abdulkhakov S, Khalikova A, et al. Serum cytokine signature that discriminates helicobacter pylori positive and negative juvenile gastroduodenitis. Front Microbiol. 2016;7:1916.
Kerami Z, Duijvis NW, Vogels EW, van Dooren FH, Moerland PD, Te Velde AA. Effect of interleukin-17 on gene expression profile of fibroblasts from Crohn’s disease patients. J Crohns Colitis. 2014;810:1208–16.
Alzoghaibi MA, Al-Mofleh IA, Al-Jebreen AM. Neutrophil chemokines GCP-2 and GRO-alpha in patients with inflammatory bowel disease. J Dig Dis. 2008;93:144–8.
Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;2134(Suppl):S29-52.
Mittal P, Romero R, Kusanovic JP, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;603:246–57.
Grad S, Bow C, Karppinen J, et al. Systemic blood plasma CCL5 and CXCL6: potential biomarkers for human lumbar disc degeneration. Eur Cell Mater. 2016;31:1–10.
Plemmenos G, Evangeliou E, Polizogopoulos N, Chalazias A, Deligianni M, Piperi C. Central regulatory role of cytokines in periodontitis and targeting options. Curr Med Chem. 2021;2815:3032–58.
Kebschull M, Demmer R, Behle JH, et al. Granulocyte chemotactic protein 2 (gcp-2/cxcl6) complements interleukin-8 in periodontal disease. J Periodontal Res. 2009;444:465–71.
Yücel Ç, Fırat Oğuz E, Er S, Balamir İ, Turhan T, Tez M. Diagnostic value of GCP-2/CXCL-6 and hs-CRP in the diagnosis of acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2020;262:191–6.
Hasegawa M, Higashi K, Matsushita T, et al. Dermokine inhibits ELR(+)CXC chemokine expression and delays early skin wound healing. J Dermatol Sci. 2013;701:34–41.
Traves SL, Donnelly LE. Th17 cells in airway diseases. Curr Mol Med. 2008;85:416–26.
Saini C, Srivastava RK, Kumar P, Ramesh V, Sharma A. A distinct double positive IL-17A(+)/F(+) T helper 17 cells induced inflammation leads to IL17 producing neutrophils in Type 1 reaction of leprosy patients. Cytokine. 2020;126:154873.
Vistejnova L, Safrankova B, Nesporova K, et al. Low molecular weight hyaluronan mediated CD44 dependent induction of IL-6 and chemokines in human dermal fibroblasts potentiates innate immune response. Cytokine. 2014;702:97–103.
Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;432:194–9.
Dong Y, Liu J, Xue Z, et al. Pao Pereira extract suppresses benign prostatic hyperplasia by inhibiting inflammation-associated NFκB signaling. BMC Complement Med Ther. 2020;201:150.
Bernichtein S, Pigat N, Camparo P, et al. Anti-inflammatory properties of Lipidosterolic extract of Serenoa repens (Permixon®) in a mouse model of prostate hyperplasia. Prostate. 2015;757:706–22.
Huth L, Marquardt Y, Heise R, et al. Bifonazole exerts anti-inflammatory effects in human three-dimensional skin equivalents after UVB or histamine challenge. Skin Pharmacol Physiol. 2019;326:337–43.
Laplane L, Duluc D, Larmonier N, Pradeu T, Bikfalvi A. The multiple layers of the tumor environment. Trends Cancer. 2018;412:802–9.
Song M, He J, Pan QZ, et al. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology. 2021;735:1717–35.
Zheng S, Shen T, Liu Q, et al. CXCL6 fuels the growth and metastases of esophageal squamous cell carcinoma cells both in vitro and in vivo through upregulation of PD-L1 via activation of STAT3 pathway. J Cell Physiol. 2021;2367:5373–86.
Li J, Tang Z, Wang H, et al. CXCL6 promotes non-small cell lung cancer cell survival and metastasis via down-regulation of miR-515-5p. Biomed Pharmacother. 2018;97:1182–8.
Li L, Man J, Zhao L. Hypoxia-CXCL6 axis affects arteriolar niche remodeling in acute myeloid leukemia. Exp Biol Med. 2021;2461:84–96.
Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;21010:2057–69.
Yeudall WA, Vaughan CA, Miyazaki H, et al. Gain-of-function mutant p53 upregulates CXC chemokines and enhances cell migration. Carcinogenesis. 2012;332:442–51.
Otomo R, Otsubo C, Matsushima-Hibiya Y, et al. TSPAN12 is a critical factor for cancer-fibroblast cell contact-mediated cancer invasion. Proc Natl Acad Sci U S A. 2014;11152:18691–6.
Wan J, Liu S, Sun W, et al. Ring finger protein 152-dependent degradation of TSPAN12 suppresses hepatocellular carcinoma progression. Cancer Cell Int. 2021;211:122.
Liu G, An L, Zhang H, Du P, Sheng Y. Activation of CXCL6/CXCR1/2 axis promotes the growth and metastasis of osteosarcoma cells in vitro and in vivo. Front Pharmacol. 2019;10:307.
Tiwari N, Marudamuthu AS, Tsukasaki Y, Ikebe M, Fu J, Shetty S. p53- and PAI-1-mediated induction of C-X-C chemokines and CXCR2: importance in pulmonary inflammation due to cigarette smoke exposure. Am J Physiol Lung Cell Mol Physiol. 2016;3106:L496-506.
Vicent S, Sayles LC, Vaka D, et al. Cross-species functional analysis of cancer-associated fibroblasts identifies a critical role for CLCF1 and IL-6 in non-small cell lung cancer in vivo. Cancer Res. 2012;7222:5744–56.
Lei MML, Lee TKW. Cancer-associated fibroblasts: orchestrating the crosstalk between liver cancer cells and neutrophils through the cardiotrophin-like cytokine factor 1-mediated chemokine (C-X-C motif) ligand 6/TGF-β axis. Hepatology. 2021;735:1631–3.
Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;1759:6177–89.
Zhang M, Wang G, Tao Y, Zhang H. The proinflammatory effect and molecular mechanism of IL- 17 in the intestinal epithelial cell line HT-29. J buon. 2015;201:120–7.
Shan ZG, Chen J, Liu JS, et al. Activated neutrophils polarize protumorigenic interleukin-17A-producing T helper subsets through TNF-α-B7-H2-dependent pathway in human gastric cancer. Clin Transl Med. 2021;116:e484.
Sivanathan KN, Rojas-Canales D, Grey ST, Gronthos S, Coates PT. Transcriptome profiling of IL-17A preactivated mesenchymal stem cells: a comparative study to unmodified and IFN-γ modified mesenchymal stem cells. Stem Cells Int. 2017;2017:1025820.
Liu L, Sun H, Wu S, et al. IL-17A promotes CXCR2-dependent angiogenesis in a mouse model of liver cancer. Mol Med Rep. 2019;202:1065–74.
Zhu YM, Bagstaff SM, Woll PJ. Production and upregulation of granulocyte chemotactic protein-2/CXCL6 by IL-1beta and hypoxia in small cell lung cancer. Br J Cancer. 2006;9412:1936–41.
Ferretti E, Di Carlo E, Cocco C, et al. Direct inhibition of human acute myeloid leukemia cell growth by IL-12. Immunol Lett. 2010;1332:99–105.
Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;2048:1837–47.
Ferretti E, Di Carlo E, Ognio E, et al. IL-25 dampens the growth of human germinal center-derived B-cell non Hodgkin Lymphoma by curtailing neoangiogenesis. Oncoimmunology. 2018;73:e1397249.
Corrigan CJ, Wang W, Meng Q, et al. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci U S A. 2011;1084:1579–84.
Wang W, Fan YQ, Lv Z, et al. Interleukin-25 promotes basic fibroblast growth factor expression by human endothelial cells through interaction with IL-17RB, but not IL-17RA. Clin Exp Allergy. 2012;4211:1604–14.
Li Y, Flores R, Yu A, et al. Elevated expression of CXC chemokines in pediatric osteosarcoma patients. Cancer. 2011;1171:207–17.
Wang N, Feng Y, Wang Q, et al. Neutrophils infiltration in the tongue squamous cell carcinoma and its correlation with CEACAM1 expression on tumor cells. PLoS ONE. 2014;92:e89991.
Korbecki J, Kojder K, Kapczuk P, et al. The effect of hypoxia on the expression of CXC chemokines and CXC chemokine receptors—a review of literature. Int J Mol Sci. 2021;22(2):843.
Chen Q, Liu D, Hu Z, Luo C, Zheng SL. miRNA-101-5p inhibits the growth and aggressiveness of NSCLC cells through targeting CXCL6. Onco Targets Ther. 2019;12:835–48.
Zhao M, Dong G, Meng Q, Lin S, Li X. Circ-HOMER1 enhances the inhibition of miR-1322 on CXCL6 to regulate the growth and aggressiveness of hepatocellular carcinoma cells. J Cell Biochem. 2020;12111:4440–9.
Shen W, Xie XY, Liu MR, Wang LL. MicroRNA-101-5p inhibits the growth and metastasis of cervical cancer cell by inhibiting CXCL6. Eur Rev Med Pharmacol Sci. 2019;235:1957–68.
Sun C, Li G, Liu M. A novel circular RNA, circ_0005394, predicts unfavorable prognosis and contributes to hepatocellular carcinoma progression by regulating miR-507/E2F3 and miR-515-5p/CXCL6 signaling pathways. Onco Targets Ther. 2020;13:6171–80.
Walterskirchen N, Müller C, Ramos C, et al. Metastatic colorectal carcinoma-associated fibroblasts have immunosuppressive properties related to increased IGFBP2 expression. Cancer Lett. 2022;540:215737.
Guil-Luna S, Mena R, Navarrete-Sirvent C, et al. Association of tumor budding with immune evasion pathways in primary colorectal cancer and patient-derived xenografts. Front Med. 2020;7:264.
Tian H, Huang P, Zhao Z, Tang W, Xia J. HIF-1α plays a role in the chemotactic migration of hepatocarcinoma cells through the modulation of CXCL6 expression. Cell Physiol Biochem. 2014;345:1536–46.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;6810:3645–54.
Ma JC, Sun XW, Su H, et al. Fibroblast-derived CXCL12/SDF-1α promotes CXCL6 secretion and co-operatively enhances metastatic potential through the PI3K/Akt/mTOR pathway in colon cancer. World J Gastroenterol. 2017;2328:5167–78.
Zhang C, Tang B, Hu J, et al. Neutrophils correlate with hypoxia microenvironment and promote progression of non-small-cell lung cancer. Bioengineered. 2021;121:8872–84.
Rodrigues FS, Ciccarelli FD, Malanchi I. Reflected stemness as a potential driver of the tumour microenvironment. Trends Cell Biol. 2022;32:979–87.
Bian J, Fu J, Wang X, et al. Characterization of immunogenicity of malignant cells with stemness in intrahepatic cholangiocarcinoma by single-cell RNA sequencing. Stem Cells Int. 2022;2022:3558200.
Gijsbers K, Gouwy M, Struyf S, et al. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp Cell Res. 2005;3032:331–42.
Karagiannis GS, Saraon P, Jarvi KA, Diamandis EP. Proteomic signatures of angiogenesis in androgen-independent prostate cancer. Prostate. 2014;743:260–72.
Mohammadi Najafabadi M, Shamsasenjan K, Akbarzadehlaleh P. The angiogenic chemokines expression profile of myeloid cell lines co-cultured with bone marrow-derived mesenchymal stem cells. Cell J. 2018;201:19–24.
Burrows AE, Smogorzewska A, Elledge SJ. Polybromo-associated BRG1-associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proc Natl Acad Sci U S A. 2010;10732:14280–5.
van Beijnum JR, Nowak-Sliwinska P, van Berkel M, Wong TJ, Griffioen AW. A genomic screen for angiosuppressor genes in the tumor endothelium identifies a multifaceted angiostatic role for bromodomain containing 7 (BRD7). Angiogenesis. 2017;204:641–54.
Tsou PS, Rabquer BJ, Ohara RA, et al. Scleroderma dermal microvascular endothelial cells exhibit defective response to pro-angiogenic chemokines. Rheumatology. 2016;554:745–54.
Lin ZY, Chuang WL. Pharmacologic concentrations of melatonin have diverse influence on differential expressions of angiogenic chemokine genes in different hepatocellular carcinoma cell lines. Biomed Pharmacother. 2010;6410:659–62.
Park SY, Jang WJ, Yi EY, et al. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res. 2010;482:178–84.
González-González A, González A, Rueda N, et al. Usefulness of melatonin as complementary to chemotherapeutic agents at different stages of the angiogenic process. Sci Rep. 2020;101:4790.
González-González A, González A, Rueda N, et al. Melatonin enhances the usefulness of ionizing radiation: involving the regulation of different steps of the angiogenic process. Front Physiol. 2019;10:879.
Wang X, Dai Y, Zhang X, et al. CXCL6 regulates cell permeability, proliferation, and apoptosis after ischemia-reperfusion injury by modulating Sirt3 expression via AKT/FOXO3a activation. Cancer Biol Ther. 2021;221:30–9.
Asselah T, Bièche I, Laurendeau I, et al. Liver gene expression signature of mild fibrosis in patients with chronic hepatitis C. Gastroenterology. 2005;1296:2064–75.
Besnard AG, Struyf S, Guabiraba R, et al. CXCL6 antibody neutralization prevents lung inflammation and fibrosis in mice in the bleomycin model. J Leukoc Biol. 2013;946:1317–23.
Taniguchi T, Asano Y, Nakamura K, et al. Fli1 Deficiency induces CXCL6 expression in dermal fibroblasts and endothelial cells, contributing to the development of fibrosis and vasculopathy in systemic sclerosis. J Rheumatol. 2017;448:1198–205.
Cai X, Li Z, Zhang Q, et al. CXCL6-EGFR-induced Kupffer cells secrete TGF-β1 promoting hepatic stellate cell activation via the SMAD2/BRD4/C-MYC/EZH2 pathway in liver fibrosis. J Cell Mol Med. 2018;2210:5050–61.
Wu C, Cheng D, Peng Y, et al. Hepatic BRD4 Is upregulated in liver fibrosis of various etiologies and positively correlated to fibrotic severity. Front Med. 2021;8:683506.
Sherwood J, Bertrand J, Nalesso G, et al. A homeostatic function of CXCR2 signalling in articular cartilage. Ann Rheum Dis. 2015;7412:2207–15.
Kawata K, Koga H, Tsuji K, et al. Extracellular vesicles derived from mesenchymal stromal cells mediate endogenous cell growth and migration via the CXCL5 and CXCL6/CXCR2 axes and repair menisci. Stem Cell Res Ther. 2021;121:414.
Min Y, Han S, Aae Ryu H, Kim SW. Human adipose mesenchymal stem cells overexpressing dual chemotactic gene showed enhanced angiogenic capacity in ischaemic hindlimb model. Cardiovasc Res. 2018;11410:1400–9.
Kim SW, Lee DW, Yu LH, et al. Mesenchymal stem cells overexpressing GCP-2 improve heart function through enhanced angiogenic properties in a myocardial infarction model. Cardiovasc Res. 2012;954:495–506.
Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;545:859–74.
Tang KH, Li S, Khodadadi-Jamayran A, et al. Combined inhibition of SHP2 and CXCR1/2 promotes antitumor T-cell response in NSCLC. Cancer Discov. 2022;121:47–61.
Rennard SI, Dale DC, Donohue JF, et al. CXCR2 antagonist MK-7123 A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;1919:1001–11.
O’Byrne PM, Metev H, Puu M, et al. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;410:797–806.
Acknowledgements
We are indebted to all individuals who participated in or helped with this research project.
Funding
This work was supported by funds from the National Natural Science Foundation of China (No. 82174023) and the Three-Year Action Plan for Shanghai TCM Development and Inheritance Program (ZY(2021-2023)-0401).
Author information
Authors and Affiliations
Contributions
HZ conceptualized the study. C-LD, H-XY and Y-QD wrote the original draft of the manuscript. C-LD, H-XY, Y-QD and HZ wrote, reviewed and edited the manuscript. KR and HZ contributed to verification and recommendation. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The review was written in accordance with ethical standards.
Consent for publication
All authors gave consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Article note has been updated as ‘’Chun-Lan Dai and Hong-Xuan Yang contributed equally to this work and are co-first authors’’.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, CL., Yang, HX., Liu, QP. et al. CXCL6: A potential therapeutic target for inflammation and cancer. Clin Exp Med 23, 4413–4427 (2023). https://doi.org/10.1007/s10238-023-01152-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01152-8