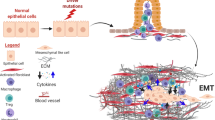
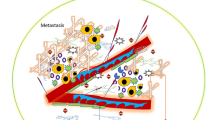
Abstract
Diagnosis and treatment of carcinoma of the exocrine part of the pancreas is a serious problem for modern medicine. Despite the identification of a large number of aberrant mutations in pancreatic ductal adenocarcinoma (PDAC), attempts to create effective therapeutic agents based on identified genetic or epigenetic variations have not been succesful. The role of the tumor microenvironment (TME) in tumor progression is currently a popular topic. Cancer-associated fibroblasts (CAFs) and extracellular matrix (ECM) are the main components of the tumor stroma and play an important role in the proliferation, invasiveness and metastasis of cancer. However, the mechanisms underlying the effect of CAFs and ECM on cancer progression are still unclear. Recent studies on stromal components and blockage of signaling pathways have brought some optimism to this area. New information on the role of TME will lead to the development of targeted therapies or combinations with modern chemotherapy for PDAC.




Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. https://doi.org/10.3322/caac.21708.
Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. https://doi.org/10.1038/nature11547.
Harris NLE, Vennin C, Conway JRW, Vine KL, Pinese M, Cowley MJ, et al. SerpinB2 regulates stromal remodelling and local invasion in pancreatic cancer. Oncogene. 2017;36:4288–98. https://doi.org/10.1038/onc.2017.63.
Haeberle L, Esposito I. Pathology of pancreatic cancer. Transl Gastroenterol Hepatol. 2019;4:50.
Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol Mech Dis. 2008;3:157–88. https://doi.org/10.1146/annurev.pathmechdis.3.121806.154305.
Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34. https://doi.org/10.1016/j.ccr.2014.04.005.
Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–20. https://doi.org/10.1136/gutjnl-2012-302529.
Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Collisson EA, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2015;159:80–93. https://doi.org/10.1016/j.cell.2014.08.007.Vitamin.
Ding C, Li Y, Xing C, Zhang H, Wang S, Dai M. Research progress on Slit/Robo pathway in pancreatic cancer: emerging and promising. J Oncol. 2020;2020:2845906. https://doi.org/10.1155/2020/2845906.
Thomas D, Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer. 2019;18:14. https://doi.org/10.1186/s12943-018-0927-5.
Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–41. https://doi.org/10.1097/PAS.0000000000000533.
Cui Zhou D, Jayasinghe RG, Chen S, Herndon JM, Iglesia MD, Navale P, et al. Spatially restricted drivers and transitional cell populations cooperate with the microenvironment in untreated and chemo-resistant pancreatic cancer. Nat Genet. 2022. https://doi.org/10.1038/s41588-022-01157-1.
Remmers N, Bailey JM, Mohr AM, Hollingsworth MA. Molecular pathology of early pancreatic cancer. Cancer Biomark. 2011;9:421–40. https://doi.org/10.3233/CBM-2011-0168.
Abe K, Suda K, Arakawa A, Yamasaki S, Sonoue H, Mitani K, et al. Different patterns of p16INK4A and p53 protein expressions in intraductal papillary-mucinous neoplasms and pancreatic intraepithelial neoplasia. Pancreas. 2007;34:85–91. https://doi.org/10.1097/01.mpa.0000240608.56806.0a.
Dal Molin M, Hong SM, Hebbar S, Sharma R, Scrimieri F, De Wilde RF, et al. Loss of expression of the SWI/SNF chromatin remodeling subunit BRG1/SMARCA4 is frequently observed in intraductal papillary mucinous neoplasms of the pancreas. Hum Pathol. 2012;43:585–91. https://doi.org/10.1016/j.humpath.2011.06.009.
Nones K, Waddell N, Song S, Patch AM, Miller D, Johns A, et al. Genome-wide DNA methylation patterns in pancreatic ductal adenocarcinoma reveal epigenetic deregulation of SLIT-ROBO, ITGA2 and MET signaling. Int J Cancer. 2014;135:1110–8. https://doi.org/10.1002/ijc.28765.
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. https://doi.org/10.1038/nature16965.
Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153–68. https://doi.org/10.1038/s41575-019-0245-4.
Raphael BJ, Hruban RH, Aguirre AJ, Moffitt RA, Yeh JJ, Stewart C, et al. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32:185-203.e13. https://doi.org/10.1016/j.ccell.2017.07.007.
Avery JT, Zhang R, Boohaker RJ. GLI1: a therapeutic target for cancer. Front Oncol. 2021;11:1833. https://doi.org/10.3389/fonc.2021.673154.
Kotoula V, Charalambous E, Biesmans B, Malousi A, Vrettou E, Fountzilas G, et al. Targeted KRAS mutation assessment on patient tumor histologic material in real time diagnostics. PLoS ONE. 2009;4:e7746. https://doi.org/10.1371/journal.pone.0007746.
Pietrobono S, Gagliardi S, Stecca B. Non-canonical hedgehog signaling pathway in cancer: activation of GLI transcription factors beyond smoothened. Front Genet. 2019;10:556. https://doi.org/10.3389/fgene.2019.00556.
Mills LD, Zhang Y, Marler RJ, Herreros-Villanueva M, Zhang L, Almada LL, et al. Loss of the transcription factor GLI1 identifies a signaling network in the tumor microenvironment mediating KRAS oncogene-induced transformation. J Biol Chem. 2013;288:11786–94. https://doi.org/10.1074/jbc.M112.438846.
Kamerkar S, Lebleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. https://doi.org/10.1038/nature22341.
Khvalevsky EZ, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20723–8. https://doi.org/10.1073/pnas.1314307110.
Golan T, Khvalevsky EZ, Hubert A, Gabai RM, Hen N, Segal A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560–70.
Asati V, Mahapatra DK, Bharti SK. K-Ras and its inhibitors towards personalized cancer treatment: Pharmacological and structural perspectives. Eur J Med Chem. 2017;125:299–314. https://doi.org/10.1016/j.ejmech.2016.09.049.
Garcia-Sampedro A, Gaggia G, Ney A, Mahamed I, Acedo P. The state-of-the-art of phase II/III clinical trials for targeted pancreatic cancer therapies. J Clin Med. 2021;10:1–45. https://doi.org/10.3390/jcm10040566.
Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. https://doi.org/10.1038/nm1087.
Hahn SA, Schutte M, Shamsul Hoque ATM, Moskaluk CA, Da Costa LT, Rozenblum E, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q211. Science (80-). 1996;271:350–3. https://doi.org/10.1126/science.271.5247.350.
Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–20. https://doi.org/10.1038/nrc1208.
Principe DR, DeCant B, Mascariñas E, Wayne EA, Diaz AM, Akagi N, et al. TGFβ signaling in the pancreatic tumor microenvironment promotes fibrosis and immune evasion to facilitate tumorigenesis. Cancer Res. 2016;76:2525–39. https://doi.org/10.1158/0008-5472.CAN-15-1293.
Takahashi K, Akatsu Y, Podyma-Inoue KA, Matsumoto T, Takahashi H, Yoshimatsu Y, et al. Targeting all transforming growth factor-b isoforms with an Fc chimeric receptor impairs tumor growth and angiogenesis of oral squamous cell cancer. J Biol Chem. 2020;295:12559–72. https://doi.org/10.1074/jbc.RA120.012492.
Nieto MA, Huang RYYJ, Jackson RAA, Thiery JPP. EMT: 2016. Cell. 2016;166:21–45. https://doi.org/10.1016/j.cell.2016.06.028.
Ahmed S, Schwartz C, Dewan M, Xu R. The promising role of TGF-β/SMAD4 in pancreatic cancer: the future targeted therapy. J Cancer Treat Diagn. 2019;3:1–7.
Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction. 2010;139:697–704. https://doi.org/10.1530/REP-10-0017.
Gohrig A, Detjen KM, Hilfenhaus G, Korner JL, Welzel M, Arsenic R, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74:1529–40. https://doi.org/10.1158/0008-5472.CAN-13-1012.
Escot S, Willnow D, Naumann H, Di Francescantonio S, Spagnoli FM. Robo signalling controls pancreatic progenitor identity by regulating Tead transcription factors. Nat Commun. 2018;9:5082. https://doi.org/10.1038/s41467-018-07474-6.
Maehara N, Matsumoto K, Kuba K, Mizumoto K, Tanaka M, Nakamura T. NK4, a four-kringle antagonist of HGF, inhibits spreading and invasion of human pancreatic cancer cells. Br J Cancer. 2001;84:864–73. https://doi.org/10.1054/bjoc.2000.1682.
Pinho AV, Van Bulck M, Chantrill L, Arshi M, Sklyarova T, Herrmann D, et al. ROBO2 is a stroma suppressor gene in the pancreas and acts via TGF-β signalling. Nat Commun. 2018. https://doi.org/10.1038/s41467-018-07497-z.
Han S, Cao C, Tang T, Lu C, Xu J, Wang S, et al. ROBO3 promotes growth and metastasis of pancreatic carcinoma. Cancer Lett. 2015;366:61–70. https://doi.org/10.1016/j.canlet.2015.06.004.
Smith R, Xue AQ, Gill A, Scarlett C, Saxby A, Clarkson A, et al. High expression of plasminogen activator inhibitor-2 (PAI-2) is a predictor of improved survival in patients with pancreatic adenocarcinoma. World J Surg. 2007;31:493–502. https://doi.org/10.1007/s00268-006-0289-9.
Kumar AA, Buckley BJ, Ranson M. The urokinase plasminogen activation system in pancreatic cancer: prospective diagnostic and therapeutic targets. Biomolecules. 2022;12:1–27. https://doi.org/10.3390/biom12020152.
LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. DMM Dis Model Mech. 2018. https://doi.org/10.1242/dmm.029447.
Vitale D, Kumar Katakam S, Greve B, Jang B, Oh ES, Alaniz L, et al. Proteoglycans and glycosaminoglycans as regulators of cancer stem cell function and therapeutic resistance. FEBS J. 2019;286:2870–82. https://doi.org/10.1111/febs.14967.
Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–34. https://doi.org/10.1038/nrclinonc.2017.101.
Obacz J, Avril T, Rubio-Patiño C, Bossowski JP, Igbaria A, Ricci JE, et al. Regulation of tumor–stroma interactions by the unfolded protein response. FEBS J. 2019;286:279–96. https://doi.org/10.1111/febs.14359.
Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17:487–505. https://doi.org/10.1038/s41575-020-0300-1.
Maishi N, Hida K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017;108:1921–6. https://doi.org/10.1111/cas.13336.
Ferrara B, Pignatelli C, Cossutta M, Citro A, Courty J, Piemonti L. The extracellular matrix in pancreatic cancer: description of a complex network and promising therapeutic options. Cancers (Basel). 2021;13:4442. https://doi.org/10.3390/cancers13174442.
Perez VM, Kearney JF, Yeh JJ. The PDAC extracellular matrix: a review of the ECM protein composition, tumor cell interaction, and therapeutic strategies. Front Oncol. 2021;11:4114. https://doi.org/10.3389/fonc.2021.751311.
Murakami T, Hiroshima Y, Matsuyama R, Homma Y, Hoffman RM, Endo I. Role of the tumor microenvironment in pancreatic cancer. Ann Gastroenterol Surg. 2019;3:130–7. https://doi.org/10.1002/ags3.12225.
Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–53. https://doi.org/10.1158/0008-5472.CAN-14-2211.
Lyssiotis CA, Kimmelman AC. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27:863–75. https://doi.org/10.1016/j.tcb.2017.06.003.
Mezawa Y, Orimo A. The roles of tumor- and metastasis-promoting carcinoma-associated fibroblasts in human carcinomas. Cell Tissue Res. 2016;365:675–89. https://doi.org/10.1007/s00441-016-2471-1.
Tian C, Clauser KR, Öhlund D, Rickelt S, Huang Y, Gupta M, et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci USA. 2019;116:19609–18. https://doi.org/10.1073/pnas.1908626116.
Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22:697–706. https://doi.org/10.1016/j.ceb.2010.08.015.
Chen Y, Yang S, Tavormina J, Tampe D, Zeisberg M, Wang H, et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. 2022. https://doi.org/10.1016/j.ccell.2022.06.011.
Afratis NA, Klepfish M, Karamanos NK, Sagi I. The apparent competitive action of ECM proteases and cross-linking enzymes during fibrosis: applications to drug discovery. Adv Drug Deliv Rev. 2018;129:4–15. https://doi.org/10.1016/j.addr.2018.03.004.
Dufort CC, Delgiorno KE, Hingorani SR. Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology. 2016;150:1545-1557.e2. https://doi.org/10.1053/j.gastro.2016.03.040.
Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 2014;310:39–87. https://doi.org/10.1016/B978-0-12-800180-6.00002-5.
Aguilera KY, Huang H, Du W, Hagopian MM, Wang Z, Hinz S, et al. Inhibition of discoidin domain receptor 1 reduces collagen-mediated tumorigenicity in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16:2473–85. https://doi.org/10.1158/1535-7163.MCT-16-0834.
DeJaime-Soguero A, AbreudeOliveira W, Lluis F. The pleiotropic effects of the canonical Wnt pathway in early development and pluripotency. Genes (Basel). 2018. https://doi.org/10.3390/genes9020093.
Xu W, Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J Cell Sci. 2007;120:3337–44. https://doi.org/10.1242/jcs.013771.
Gao C, Chen G, Kuan SF, Zhang DH, Schlaepfer DD, Hu J. FAK/PYK2 promotes the Wnt/β-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3β. Elife. 2015. https://doi.org/10.7554/eLife.10072.
González-Sancho JM, Larriba MJ, Muñoz A. Wnt and vitamin d at the crossroads in solid cancer. Cancers (Basel). 2020;12:1–19. https://doi.org/10.3390/cancers12113434.
Di Martino JS, Nobre AR, Mondal C, Taha I, Farias EF, Fertig EJ, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. 2021. https://doi.org/10.1038/s43018-021-00291-9.
Zinger A, Koren L, Adir O, Poley M, Alyan M, Yaari Z, et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano. 2019;13:11008–21. https://doi.org/10.1021/acsnano.9b02395.
Jiang H, Torphy RJ, Steiger K, Hongo H, Ritchie AJ, Kriegsmann M, et al. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J Clin Invest. 2020;130:4704–9. https://doi.org/10.1172/JCI136760.
Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins α11β1 and α2β1. J Biol Chem. 2002;277:37377–81. https://doi.org/10.1074/jbc.M206286200.
Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β 1 function. Science (80-). 2009;323:642–4. https://doi.org/10.1126/science.1168441.
Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216:3799–816. https://doi.org/10.1083/jcb.201704053.
Almokadem S, Belani CP. Volociximab in cancer. Expert Opin Biol Ther. 2012;12:251–7. https://doi.org/10.1517/14712598.2012.646985.
Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–13. https://doi.org/10.1242/jcs.045112.
Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–60. https://doi.org/10.1038/nm.4123.
Hochwald SN, Nyberg C, Zheng M, Zheng D, Wood C, Massoll NA, et al. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle. 2009;8:2435–43. https://doi.org/10.4161/cc.8.15.9145.
Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to- mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–7. https://doi.org/10.1016/j.ceb.2005.08.007.
Ridgway RA, Serrels B, Mason S, Kinnaird A, Muir M, Patel H, et al. Focal adhesion kinase is required for β-catenin-induced mobilization of epidermal stem cells. Carcinogenesis. 2012;33:2369–76. https://doi.org/10.1093/carcin/bgs284.
Zheng D, Duan H, Wang S, Xu Q, Gan L, Li J, et al. FAK regulates epithelial-mesenchymal transition in adenomyosis. Mol Med Rep. 2018;18:5461–72. https://doi.org/10.3892/mmr.2018.9600.
Viloria K, Hill NJ. Embracing the complexity of matricellular proteins: The functional and clinical significance of splice variation. Biomol Concepts. 2016;7:117–32. https://doi.org/10.1515/bmc-2016-0004.
Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–30. https://doi.org/10.1038/sj.onc.1206807.
Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, et al. Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. DMM Dis Model Mech. 2010;3:57–72. https://doi.org/10.1242/dmm.003228.
Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol. 2015;6:201. https://doi.org/10.3389/fimmu.2015.00201.
Sato N, Cheng XB, Kohi S, Koga A, Hirata K. Targeting hyaluronan for the treatment of pancreatic ductal adenocarcinoma. Acta Pharm Sin B. 2016;6:101–5. https://doi.org/10.1016/j.apsb.2016.01.002.
Caon I, Bartolini B, Parnigoni A, Caravà E, Moretto P, Viola M, et al. Revisiting the hallmarks of cancer: the role of hyaluronan. Semin Cancer Biol. 2020;62:9–19. https://doi.org/10.1016/j.semcancer.2019.07.007.
Cheng XB, Sato N, Kohi S, Koga A, Hirata K. Receptor for hyaluronic acid-mediated motility is associated with poor survival in pancreatic ductal adenocarcinoma. J Cancer. 2015;6:1093–8. https://doi.org/10.7150/jca.12990.
Molejon MI, Tellechea JI, Loncle C, Gayet O, Gilabert M, Duconseil P, et al. Deciphering the cellular source of tumor relapse identifies CD44 as a major therapeutic target in pancreatic adenocarcinoma. Oncotarget. 2015;6:7408–23.
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. https://doi.org/10.1016/j.ccr.2012.01.007.
Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, et al. HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36:359–66. https://doi.org/10.1200/JCO.2017.74.9564.
Ramanathan RK, McDonough SL, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol. 2019;37:1062–9. https://doi.org/10.1200/JCO.18.01295.
Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108:2909–14. https://doi.org/10.1073/pnas.1018892108.
Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. https://doi.org/10.1038/ncomms3516.
Liu H, Naxerova K, Pinter M, Incio J, Lee H, Shigeta K, et al. Use of angiotensin system inhibitors is associated with immune activation and longer survival in nonmetastatic pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23:5959–69. https://doi.org/10.1158/1078-0432.CCR-17-0256.
Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with Losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–7. https://doi.org/10.1001/jamaoncol.2019.0892.
George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10:745–59. https://doi.org/10.1038/nrc2945.
Buchholz M, Kestler HA, Holzmann K, Ellenrieder V, Schneiderhan W, Siech M, et al. Transcriptome analysis of human hepatic and pancreatic stellate cells: Organ-specific variations of a common transcriptional phenotype. J Mol Med. 2005;83:795–805. https://doi.org/10.1007/s00109-005-0680-2.
Watari N, Hotta Y, Mabuchi Y. Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration. Okajimas Folia Anat Jpn. 1982;58:837–57. https://doi.org/10.2535/ofaj1936.58.4-6_837.
Bachem MG, Zhou S, Buck K, Schneiderhan W, Siech M. Pancreatic stellate cells—role in pancreas cancer. Langenbeck’s Arch Surg. 2008;393:891–900. https://doi.org/10.1007/s00423-008-0279-5.
Maeda K, Enomoto A, Hara A, Asai N, Kobayashi T, Horinouchi A, et al. Identification of Meflin as a potential marker for mesenchymal stromal cells. Sci Rep. 2016;6:22288. https://doi.org/10.1038/srep22288.
Takahashi M, Kobayashi H, Mizutani Y, Hara A, Iida T, Miyai Y, et al. Roles of the mesenchymal stromal/stem cell marker Meflin/Islr in cancer fibrosis. Front Cell Dev Biol. 2021;9:2687. https://doi.org/10.3389/fcell.2021.749924.
Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, et al. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. 2019;79:5367–81. https://doi.org/10.1158/0008-5472.CAN-19-0454.
Musa M. Single-cell analysis on stromal fibroblasts in the microenvironment of solid tumours. Adv Med Sci. 2020;65:163–9. https://doi.org/10.1016/j.advms.2019.12.001.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. https://doi.org/10.1038/nrc.2016.73.
Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel). 2015;7:2443–58. https://doi.org/10.3390/cancers7040902.
Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–9. https://doi.org/10.1038/sj.bjc.6604662.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. https://doi.org/10.1172/JCI39104.
Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, et al. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780–94. https://doi.org/10.1016/S0016-5085(00)70148-X.
Jaster R. Molecular regulation of pancreatic stellate cell function. Mol Cancer. 2004;3:26. https://doi.org/10.1186/1476-4598-3-26.
Marzoq AJ, Mustafa SA, Heidrich L, Hoheisel JD, Alhamdani MSS. Impact of the secretome of activated pancreatic stellate cells on growth and differentiation of pancreatic tumour cells. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-41740-x.
Jin G, Hong W, Guo Y, Bai Y, Chen B. Molecular mechanism of pancreatic stellate cells activation in chronic pancreatitis and pancreatic cancer. J Cancer. 2020;11:1505–15. https://doi.org/10.7150/jca.38616.
Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–96. https://doi.org/10.2353/ajpath.2010.090899.
Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–6. https://doi.org/10.4161/cbt.5.12.3354.
Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, et al. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009;69:369–78. https://doi.org/10.1158/0008-5472.CAN-08-2724.
Zhu J, Thakolwiboon S, Liu X, Zhang M, Lubman DM. Overexpression of cd90 (thy-1) in pancreatic adenocarcinoma present in the tumor microenvironment. PLoS ONE. 2014;9:e115507. https://doi.org/10.1371/journal.pone.0115507.
Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257–66. https://doi.org/10.1158/1535-7163.MCT-11-0340.
De Jaeghere EA, Denys HG, De Wever O. Fibroblasts fuel immune escape in the tumor microenvironment. Trends in Cancer. 2019;5:704–23. https://doi.org/10.1016/j.trecan.2019.09.009.
Bernard V, Semaan A, Huang J, AnthonySanLucas F, Mulu FC, Stephens BM, et al. Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin Cancer Res. 2019;25:2194–205. https://doi.org/10.1158/1078-0432.CCR-18-1955.
Avery D, Govindaraju P, Jacob M, Todd L, Monslow J, Puré E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol. 2018;67:90–106. https://doi.org/10.1016/j.matbio.2017.12.003.
Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–96. https://doi.org/10.1084/jem.20162024.
Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, et al. Il1-induced Jak/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9:282–301. https://doi.org/10.1158/2159-8290.CD-18-0710.
Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102–23. https://doi.org/10.1158/2159-8290.CD-19-0094.
Chen Y, Kim J, Yang S, Wang H, Wu C-J, Sugimoto H, et al. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 2021. https://doi.org/10.1016/j.ccell.2021.02.007.
Hurwitz H, Van Cutsem E, Bendell J, Hidalgo M, Li CP, Salvo MG, et al. Ruxolitinib + capecitabine in advanced/metastatic pancreatic cancer after disease progression/intolerance to first-line therapy: JANUS 1 and 2 randomized phase III studies. Invest New Drugs. 2018;36:683–95. https://doi.org/10.1007/s10637-018-0580-2.
Ostapoff KT, Cenik BK, Wang M, Ye R, Xu X, Nugent D, et al. Neutralizing murine TGFβR2 promotes a differentiated tumor cell phenotype and inhibits pancreatic cancer metastasis. Cancer Res. 2014;74:4996–5007. https://doi.org/10.1158/0008-5472.CAN-13-1807.
Mortezaee K. CXCL12/CXCR4 axis in the microenvironment of solid tumors: a critical mediator of metastasis. Life Sci. 2020;249:117534. https://doi.org/10.1016/j.lfs.2020.117534.
Saftoiu A, Angelescu R, Burada F, Angelescu C, Gheonea D, Iordache S, et al. Expression of vascular endothelial growth factor and epidermal growth factor receptor in pancreatic ductal adenocarcinomas, neuroendocrine tumours and chronic pancreatitis. Endosc Ultrasound. 2013;2:86. https://doi.org/10.4103/2303-9027.117692.
Aoyagi Y, Oda T, Kinoshita T, Nakahashi C, Hasebe T, Ohkohchi N, et al. Overexpression of TGF-β by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. Br J Cancer. 2004;91:1316–26. https://doi.org/10.1038/sj.bjc.6602141.
Masamune A, Kikuta K, Satoh M, Kume K, Shimosegawa T. Differential roles of signaling pathways for proliferation and migration of rat pancreatic stellate cells. Tohoku J Exp Med. 2003;199:69–84. https://doi.org/10.1620/tjem.199.69.
Datta J, Dai X, Bianchi A, De Castro SI, Mehra S, Garrido V, et al. Combined MEK and STAT3 inhibition uncovers stromal plasticity by enriching for cancer-associated fibroblasts with mesenchymal stem cell-like features to overcome immunotherapy resistance in pancreatic cancer. Gastroenterology. 2022. https://doi.org/10.1053/j.gastro.2022.07.076.
Maitra A. Tracking down the Hedgehog’s lair in the pancreas. Gastroenterology. 2010;138:823–5. https://doi.org/10.1053/j.gastro.2010.01.021.
Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–47. https://doi.org/10.1016/j.ccr.2014.04.021.
Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. https://doi.org/10.1158/1078-0432.CCR-08-0291.
Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–96. https://doi.org/10.1158/0008-5472.CAN-06-3281.
Khan S, Ebeling MC, Chauhan N, Thompson PA, Gara RK, Ganju A, et al. Ormeloxifene suppresses desmoplasia and enhances sensitivity of gemcitabine in pancreatic cancer. Cancer Res. 2015;75:2292–304. https://doi.org/10.1158/0008-5472.CAN-14-2397.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res. 2014;20:5937–45. https://doi.org/10.1158/1078-0432.CCR-14-1269.
Catenacci DVT, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284–92. https://doi.org/10.1200/JCO.2015.62.8719.
Masamune A, Kikuta K, Satoh M, Sakai Y, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-γ block activation of pancreatic stellate cells. J Biol Chem. 2002;277:141–7. https://doi.org/10.1074/jbc.M107582200.
Arensman MD, Nguyen P, Kershaw KM, Lay AR, Ostertag-Hill CA, Sherman MH, et al. Calcipotriol targets LRP6 to inhibit wnt signaling in pancreatic cancer. Mol Cancer Res. 2015;13:1509–19. https://doi.org/10.1158/1541-7786.MCR-15-0204.
LaRocca C, Warner S. A new role for vitamin D: the enhancement of oncolytic viral therapy in pancreatic cancer. Biomedicines. 2018;6:104. https://doi.org/10.3390/biomedicines6040104.
Iida T, Mizutani Y, Esaki N, Ponik SM, Burkel BM, Weng L, et al. Correction: Pharmacologic conversion of cancer-associated fibroblasts from a protumor phenotype to an antitumor phenotype improves the sensitivity of pancreatic cancer to chemotherapeutics. Oncogene. 2022;41:3302–3302. https://doi.org/10.1038/s41388-022-02336-4.
Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, et al. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68:159–71. https://doi.org/10.1136/gutjnl-2018-316451.
Knudsen ES, Vail P, Balaji U, Ngo H, Botros IW, Makarov V, et al. Stratification of pancreatic ductal adenocarcinoma: combinatorial genetic, stromal, and immunologic markers. Clin Cancer Res. 2017;23:4429–40. https://doi.org/10.1158/1078-0432.CCR-17-0162.
Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–3. https://doi.org/10.1038/nm.2344.
Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SGH, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–78. https://doi.org/10.1038/ng.3398.
Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology. 2018;155:1999-2013.e3. https://doi.org/10.1053/j.gastro.2018.08.033.
Wang X, Li L, Yang Y, Fan L, Ma Y, Mao F. Reveal the heterogeneity in the tumor microenvironment of pancreatic cancer and analyze the differences in prognosis and immunotherapy responses of distinct immune subtypes. Front Oncol. 2022;12:318. https://doi.org/10.3389/fonc.2022.832715.
O’Kane GM, Grunwald BT, Jang GH, Masoomian M, Picardo S, Grant RC, et al. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin Cancer Res. 2020;26:4901–10. https://doi.org/10.1158/1078-0432.CCR-19-3724.
Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248:51–65. https://doi.org/10.1002/path.5224.
Fabris L, Perugorria MJ, Mertens J, Björkström NK, Cramer T, Lleo A, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39:63–78. https://doi.org/10.1111/liv.14098.
Acknowledgements
The work was conducted under the Inner Grant of Evdokimov Moscow State University of Medicine and Dentistry and the IDB RAS Government Basic Research Program in 2022 No 0088-2021-0017.
Funding
Nospecial funding was received by the authors of this review.
Author information
Authors and Affiliations
Contributions
AI conceived the presented idea. Literature search and data analysis were performed by AK, OP and TD. AK and AI prepared the figures. The first draft of the manuscript was written by AK. OP, AI and DP drafted and critically revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
The authors give their consent to publish this review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuznetsova, A., Popova, O., Panchenkov, D. et al. Pancreatic ductal adenocarcinoma: tumor microenvironment and problems in the development of novel therapeutic strategies. Clin Exp Med 23, 619–643 (2023). https://doi.org/10.1007/s10238-022-00886-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00886-1