Abstract
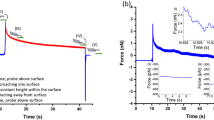
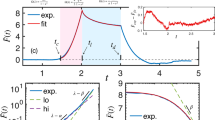
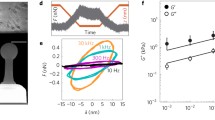
Study of the dynamic evolutions of cell viscoelasticity is important as during cell activities such as cell metastasis and invasion, the rheological behaviors of the cells also change dynamically, reflecting the biophysical and biochemical connections between the outer cortex and the intracellular structures. Although the time variations of the static modulus of cells have been investigated, few studies have been reported on the dynamic variations of the frequency-dependent viscoelasticity of cells. Measuring and monitoring such dynamic evolutions of cells at nanoscale can be challenging as the measurement needs to meet two objectives inherently contradictory to each other—the measurement must be broadband (to cover a large frequency spectrum) but also rapid (to capture the time-elapsed changes). In this study, we exploited a recently developed control-based nanomechanical protocol of atomic force microscope to monitor in real time the dynamic evolutions of the viscoelasticity of live human prostate cancer cells (PC-3 cells) and study its dependence on myosin activities. We found that the viscoelasticity of PC-3 cells, followed the power law, and oscillated at a period of about 200 s. Both the amplitude and the frequency of the oscillation strongly depended on the intracellular calcium and blebbistatin-sensitive motor proteins.









Similar content being viewed by others
References
Abidine Y, Laurent VM, Michel R, Duperray A, Palade LI, Verdier C (2015) Physical properties of polyacrylamide gels probed by AFM and rheology. EPL (Europhys Lett) 109(3):38003
Abidine Y, Laurent VM, Michel R, Duperray A, Verdier C (2015) Local mechanical properties of bladder cancer cells measured by AFM as a signature of metastatic potential. EPJPlus 130:202
Alcaraz J, Buscemi L, Grabulosa M, Trepat X, Fabry B, Farré R, Navajas D (2003) Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys J 84(3):2071–2079
Alonso J, Goldmann W (2003) Feeling the forces: atomic force microscopy in cell biology. Life Sci 72(23):2553–2560
Berret JF (2016) Local viscoelasticity of living cells measured by rotational magnetic spectroscopy. Nat Commun 7:10134
Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E (2006) Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell 11(5):613–627
Butt HJ, Cappella B, Kappl M (2005) Force measurements with the atomic force microscope: technique, interpretation and applications. Surf Sci Rep 59:1–152
Chaudhuri O, Parekh SH, Fletcher DA (2007) Reversible stress softening of actin networks. Nature 445(7125):295–298
Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Käs J, Ulvick S, Bilby C (2005) Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J 88:3689–3698
He L, Wang X, Tang HL, Montell DJ (2010) Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol 12(12):1133–1142
Hoffman BD, Crocker JC (2009) Cell mechanics: dissecting the physical responses of cells to force. Ann Rev Biomed Eng 11:259–288
Hoffman BD, Massiera G, Van Citters KM, Crocker JC (2006) The consensus mechanics of cultured mammalian cells. Proc Natl Acad Sci 103(27):10259–10264
Kim K, Zou Q (2013) A modeling-free inversion-based iterative feedforward control for precision output tracking of linear time-invariant systems. IEEE/ASME Trans Mech 18(6):1767–1777
Kovács M, Tóth J, Hetényi C, Málnási-Csizmadia A, Sellers JR (2004) Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279(34):35557–35563
Lekka M (2016) Discrimination between normal and cancerous cells using AFM. BioNanoScience 6(1):65–80
Lekka M, Laidler P (2009) Applicability of afm in cancer detection. Nat Nanotechnol 4(2):72–72
Lenormand G, Millet E, Fabry B, Butler JP, Fredberg JJ (2004) Linearity and time-scale invariance of the creep function in living cells. J R Soc Interface 1(1):91–97
Li Q, Lee G, Ong C, Lim C (2008) AFM indentation study of breast cancer cells. Biochem Biophys Res Commun 374:609–613
Lytton J, Westlin M, Hanley MR (1991) Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum CA-ATPase family of calcium pumps. J Biol Chem 266(26):17067–17071
Mason TG, Weitz D (1995) Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys Rev Lett 74(7):1250
Moore TM, Brough GH, Babal P, Kelly JJ, Li M, Stevens T (1998) Store-operated calcium entry promotes shape change in pulmonary endothelial cells expressing Trp1. Am J Phys Lung Cell Mol Phys 275(3):L574–L582
Neuman K, Nagy A (2008) Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods 5(6):491–505
Radmacher M, Tillmann R, Gaub H (1993) Imaging viscoelasticity by force modulation with the atomic force microscope. Biophys J 64(3):735–742
Ren J, Huang H, Liu Y, Zheng X, Zou Q (2015) An atomic force microscope study revealed two mechanisms in the effect of anticancer drugs on rate-dependent young’s modulus of human prostate cancer cells. PloS one 10(5):e0126107
Ren J, Mousavi A, Li X, Zou Q, Erina N, Su C (2013a) Enhanced measurement of broadband nanomechanical property of polymers using atomic force microscope. Appl Phys Lett 102(18):183116–183116
Ren J, Yu S, Gao N, Zou Q (2013b) Indentation quantification for in-liquid nanomechanical measurement of soft material using an atomic force microscope: rate-dependent elastic modulus of live cells. Phys Rev E 88(5):052711
Ren J, Zou Q (2014) A control-based approach to accurate nanoindentation quantification in broadband nanomechanical measurement using scanning probe microscope. IEEE Trans Nanotechnol 13(1):46–54
Rother J, Nöding H, Mey I, Janshoff A (2014) Atomic force microscopy-based microrheology reveals significant differences in the viscoelastic response between malign and benign cell lines. Open Biol 4(5):140046
Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP (2006) Force sensing by mechanical extension of the src family kinase substrate p130cas. Cell 127(5):1015–1026
Schillers H, Wälte M, Urbanova K, Oberleithner H (2010) Real-time monitoring of cell elasticity reveals oscillating myosin activity. Biophys J 99(11):3639–3646
Shroff SG, Saner DR, Lal R (1995) Dynamic micromechanical properities of cultured rat atrial myocytes measured by atomic force microscopy. Am J Phys 269:C286–C292
Thastrup O, Dawson A, Scharff O, Foder B, Cullen P, Drøbak B, Bjerrum P, Christensen S, Hanley M (1989) Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Inflam Res 27(1):17–23
Van Citters KM, Hoffman BD, Massiera G, Crocker JC (2006) The role of f-actin and myosin in epithelial cell rheology. Biophys J 91(10):3946–3956
Wahl K, Asif S, Greenwoodc J, Johnson K (2006) Oscillating adhesive contacts between micron-scale tips and compliant polymers. J Colloid Interface Sci 296:178–188
Weihs D, Mason TG, Teitell MA (2006) Bio-microrheology: a frontier in microrheology. Biophys J 91(11):4296–4305
Wirtz D, Konstantopoulos K, Searson PC (2011) The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11(7):512–522
Yamada S, Wirtz D, Kuo SC (2000) Mechanics of living cells measured by laser tracking microrheology. Biophys J 78:1736–1747
Yan B, Ren J, Liu Y, Huang H, Zheng X, Zou Q (2016) Study of cholesterol repletion effect on nanomechanical properties of human umbilical vein endothelial cell via rapid broadband atomic force microscopy. J Biomech Eng 139(3):034501
Yang N, Wong KKH, de Bruyn JR, Hutter JL (2008) Frequency-dependent viscoelasticity measurement by atomic force microscopy. Meas Sci Technol 20(2):025703
Zou Q, Ren J (2015) US patent: method and apparatus for nanomechancial measurement using an atomic force microscope. Approved
Acknowledgements
Ren’s and Zou’s part of work in this project was funded by NSF Grant DBI-1353890, and Yan’s part of the work was supported by the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
BY performed the AFM experiment and participated in the data analysis, JR participated in the experiment and the maniscript writing and performed the data analysis, XZ participated in the design of the experiment and guided the sample preparation and treatment, YL performed the cell sample preparation and treatment, and QZ designed the experiment, participated in the data analysis, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Bo Yan and Juan Ren were with Zou’s group, and contributed equally during this work.
Rights and permissions
About this article
Cite this article
Yan, B., Ren, J., Zheng, X. et al. High-speed broadband monitoring of cell viscoelasticity in real time shows myosin-dependent oscillations. Biomech Model Mechanobiol 16, 1857–1868 (2017). https://doi.org/10.1007/s10237-017-0924-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0924-4