Abstract
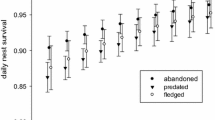
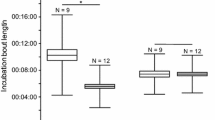
Egg predation is a common feature influencing the reproductive success of open nesting birds. Evolutionary pressure therefore favours building cryptic, inconspicuous nests. However, these antipredatory pressures may be in conflict with thermoregulatory constraints, which select for dry nest material maintaining optimum temperature inside a nest cup during the absence of incubating parents. Here we examined possible trade-offs between nest crypsis and thermoregulation in Little Grebes (Tachybaptus ruficollis), which lay their eggs in floating nests built from wet plant material. As this species regularly covers its eggs with nest material, we experimentally examined (1) the rates of egg predation on covered and uncovered artificial nests and (2) possible thermoregulatory costs from nest covering by comparing temperature and relative humidity changes inside the nest cup. Results revealed that covering clutches is beneficial in terms of deterring predators, because uncovered eggs were more vulnerable to predation. Moreover, covering clutches also had thermoregulatory benefits because the mean temperature and relative humidity inside nest cups covered by dry or wet materials were significantly higher for covered compared to uncovered treatments. Covering clutches in Little Grebes therefore does not pose thermoregulatory costs.


Similar content being viewed by others
References
Ackerman JT, Blackmer AL, Eadie JM (2004) Is predation on waterfowl nests density dependent? Tests at three spatial scales. Oikos 107:128–140
Agresti A (1992) A survey of exact inference for contigency tables. Stat Sci 7:131–153
Albrecht T, Klvaňa P (2004) Nest crypsis, reproductive value of a clutch and escape decisions in incubating female mallards (Anas platyrhynchos). Ethology 110:603–613
Cave AJ, Visser J, Perdeck AC (1989) Size and quality of the Coot (Fulica atra) territory in relation to age of its tenants and neighbours. Ardea 77:87–97
Clark RG, Shutler D (1999) Avian habitat selection: pattern from process in nest-site use by ducks? Ecology 80:272–287
Collias NE (1997) On the origin and evolution of nest building by passerine birds. Condor 99:253–270
Collias NE, Collias EC (1984) Nest building and bird behaviour. Princeton University Press, Princeton
Colombelli-Negrel D, Kleindorfer S (2009) Nest height, nest concealment, and predator type predict nest predation in superb-fairy wrens (Malurus cyaneus). Ecol Res 24:921–928
Cramp S, Simmons KEL (eds) (1977) Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic, vol I. Ostrich to ducks. Oxford University Press, Oxford, p 722
Cresswell W (1997) Nest predation: the relative effects of nest characteristics, clutch size and parental behaviour. Anim Behav 53:93–103
Flaspohler DJ, Temple SA, Rosenfield RN (2000) Relationship between nest success and concealment in two ground-nesting passerines. J Field Ornithol 71:736–747
Franklin DC (1995) Helmeted honeyeaters build bulkier nests in cold weather. Auk 112:247–248
Glutz von Blotzheim UN, Bauer KM, Bezzel E (1987) Handbuch der Vogel Mitteleuropas. Band 1. Gaviformes––Phoenicopteriformes. AULA, Frankfurt am Main
Götmark F (1993) Conspicuous nests may select for non-cryptic eggs––a comparative study of avian families. Ornis Fenn 70:102–105
Götmark F, Ahlund M (1984) Do field observers attract nest predators and influence nesting success of common eiders? J Wildl Manage 48:381–387
Hansell M (1996) The function of lichen flakes and white spider cocoons on the outer surface of birds’ nests. J Nat Hist 30:303–311
Hansell M (2000) Bird nests and construction behavior. Cambridge University Press, London
Healy S, Walsh P, Hansell M (2008) Nest building by birds. Curr Biol 18:R271–R273
Hilton GM, Hansell MH, Ruxton GD, Reid JM, Monaghan P (2004) Using artificial nests to test importance of nesting material and nest shelter for incubation energetics. AUK 121:777–787
Hoi H, Winkler H (1994) Predation on nests: a case of apparent competition. Oecologia 87:436–440
Jones D, Dekker R, Roselaar C (1995) The megapodes. Oxford University Press, New York
Kilner RM (2006) The evolution of egg colour and patterning in birds. Biol Rev 81:383–406
Kreisinger J, Albrecht T (2008) Nest protection in mallards Anas platyrhynchos: untangling the role of crypsis and parental behaviour. Funct Ecol 22:872–879
Low M (2004) Female weight predicts the timing of forced copulation attempts in stitchbirds, Notiomystis cincta. Anim Behav 68:637–644
Martin E (1992) Breeding productivity considerations: what are the appropriate habitat features for management? In: Hagan JM III, Johnston DW (eds) Ecology, conservation of neotropical migrant landbirds. Smithsonian Institution Press, Washington, DC, pp 455–473
Martin TE (1993) Nest predation and nest sites: new perspectives and old patterns. Bioscience 43:523–532
Martin TE, Scott J, Menge C (2000) Nest predation increases with parental activity: separating nest site and parental activity effects. Proc R Soc Lond B Biol Sci 267:2287–2293
Mayer PM, Smith LM, Ford RG, Watterson DC, McCutchen MD, Ryan MR (2009) Nest construction by a ground-nesting bird represents a potential trade-off between egg crypticity and thermoregulation. Oecologia 159:893–901
Mickelson PG (1975) Breeding biology of cackling geese and associated species on the Yukon-Kuskokwim Delta, Alaska. Wildl Monogr 45:3–35
Montgomerie RD, Weatherhead PJ (1988) Risks and rewards of nest defence by parent birds. Q Rev Biol 63:167–187
Opermanis O (2004) Appearance and vulnerability of artificial duck nests to avian predators. J Avian Biol 35:410–415
Ricklefs RE (1969) An analysis of nesting mortality in birds. Smithson Contrib Zool 9:1–48
Salonen V, Penttinen A (1988) Factors affecting nest predation in the great crested grebe––field observations, experiments and their statistical analysis. Ornis Fenn 65:13–20
Schmidt KA, Whelan CJ (1999) Nest predation on woodland songbirds: when is nest predation density dependent? Oikos 87:65–74
Schuetz JG (2005) Common waxbills use carnivore scat to reduce the risk of nest predation. Behav Ecol 16:133–137
Summers RW, Hockey PAR (1981) Egg-covering behavior of the white fronted plover (Charadrius marginatus). Ornis Scand 12:240–243
Szentirmai I, Székely T, Liker A (2005) The influence of nest size on heat loss in penduline tit eggs. Acta Zool Acad Sci Hung 51:59–66
Trnka A (1999) Birds of fishponds in northwestern part of Podunajská nížina lowland, Part I. Trnava University Press, Trnava, p 95
Underhill-Day JC (1985) The food of breeding Marsh Harriers Circus aeruginosus in East Anglia. Bird Study 32:199–206
Vacca MM, Handel C (1988) Factors influencing predation associated with visits to artificial goose nests. J Field Ornithol 59:215–223
Valera F, Hoi H, Schleicher B (1997) Egg burial in penduline tits, Remiz pendulinus: its role in mate desertion and female polyandry. Behav Ecol 8:20–27
Weidinger K (2004) Relative effects of nest size and site on the risk of predation in open nesting passerines. J Avian Biol 35:515–523
White W, Kennedy ED (1997) Effect of egg covering and habitat on nest destruction by house wrens. Condor 99:873–879
Acknowledgments
We would like to thank Tomáš Albrecht for helpful comments on an earlier draft of the manuscript. We also would like to thank two anonymous referees for their constructive comments. The study was partially supported by the Slovak Grant Agency for Science VEGA, project no. 1/0566/09.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Prokop, P., Trnka, A. Why do grebes cover their nests? Laboratory and field tests of two alternative hypotheses. J Ethol 29, 17–22 (2011). https://doi.org/10.1007/s10164-010-0214-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-010-0214-4