Abstract
Background
Kidney transplant patients have lower antibody acquisition after SARS-CoV-2 vaccination. The efficacy of vaccines in Japanese kidney transplant patients with specific characteristics, such as predominant living-donor, ABO-incompatible kidney transplant, and low-dose immunosuppression, requires verification.
Methods
We conducted a prospective study to estimate anti-SARS-CoV-2 antibody levels in 105 kidney transplant patients and 57 controls. Blood samples were obtained before vaccination, 1, 3, and 6 months after second vaccination, and 1 month after third vaccination. We investigated antibody acquisition rates, antibody levels, and factors associated with antibody acquisition.
Results
One month after second vaccination, antibody acquisition was 100% in the controls but only 36.7% in the kidney transplant group (P < 0.001). Antibody levels in positive kidney transplant patients were also lower than in the controls (median, 4.9 arbitrary units vs 106.4 arbitrary units, respectively, P < 0.001). Years after kidney transplant (odds ratio 1.107, 95% confidence interval 1.012–1.211), ABO-incompatible kidney transplant (odds ratio 0.316, 95% confidence interval 0.101–0.991) and mycophenolate mofetil use (odds ratio 0.177, 95% confidence interval 0.054–0.570) were significant predictors for antibody acquisition after second vaccination. After third vaccination, antibody positivity in the kidney transplant group increased to 75.3%, and antibody levels in positive patients were 71.7 arbitrary units. No factors were associated with de novo antibody acquisition.
Conclusions
In Japanese kidney transplant patients, years after kidney transplant, ABO-incompatible kidney transplant and mycophenolate mofetil use were predictors for antibody acquisition after second vaccination. Third vaccination improves antibody status even in patients who were seronegative after the second vaccination.
Similar content being viewed by others
Introduction
The outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China, and became a serious worldwide pandemic. By December 2022, it had infected approximately 650 million people and 6.6 million had died. The development of vaccines and therapeutic drugs has rapidly progressed in response to the pandemic. In 2021, two major SARS-CoV-2 mRNA vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), were launched. In Japan, BNT162b2 was granted emergency use authorization in February 2021, and mRNA-1273 was authorized in May 2021. By December 2022, more than 80% of the population had completed two vaccinations, and 67% had received a third. Substantial antibody reactions were observed after two vaccinations, and studies from various countries demonstrated high antibody acquisition rates and antibody levels [1,2,3,4]. In Japan, the antibody acquisition rate was reported to be 99.5% [5].
However, in patients with specific underlying diseases, severe COVID-19 disease is more likely, and response to vaccination is reduced. Chronic kidney disease (CKD), dialysis, and kidney transplantation (KT) are important underlying factors. The incidence rate ratio of death in subjects with CKD and COVID-19 was reported to be 10.26-fold higher than in individuals with CKD without COVID-19 [6]. CKD and KT patients, and those undergoing dialysis, are prioritized for vaccination. Dialysis patients show high antibody acquisition rates and low antibody levels [7,8,9]. In KT patients, antibody acquisition rates are remarkably low at 4–48% [10]. Because COVID-19 is a global issue, it is important to verify the safety and efficacy of vaccines in all countries and ethnic groups. Furthermore, there are several differences in KT clinical practice between Japan and other countries regarding living-donor KT, ABO-incompatible (ABO-I) KT, and use of a lower maintenance dose of immunosuppressant [11].
In this study, we investigated the antibody response after the second and third SARS-CoV-2 mRNA vaccinations in Japanese KT patients and identified factors associated with the antibody response in this population.
Patients and methods
Study design and population
This was a prospective study in 105 adult KT patients at outpatient clinics of Fukuoka University Hospital and Hara-Sanshin Hospital. Patients who had received KT within one year were excluded because they were on higher doses of immunosuppressive agents and had a high frequency of infection and rejection that could require anti-rejection therapy or modifying immunosuppressive regimens. Patients who experienced acute infection or rejection episodes within the 6 months before the study were also excluded. The control group included 57 hospital staff members matched by age, sex, and body mass index (BMI). We obtained demographic and clinical characteristics, KT-specific information, medical history, and complications after KT. Both KT and control groups initially received a series of two SARS-CoV-2 mRNA vaccinations, either BNT162b2 or mRNA-1273. Subjects who developed COVID-19 or received SARS-CoV-2 vaccine before baseline blood sampling were excluded. Blood samples were obtained before vaccination (M0), and 1 (M1), 3 (M3), and 6 months (M6) after vaccination. We defined M1 sampling as 14–56 days, M3 as 80–120 days, and M6 as 150–210 days after the second vaccination. Because 87 KT patients and 50 controls received a third vaccination approximately 6 months after the second as per Ministry of Health, Labour, and Welfare recommendations, we further sampled at 1 month after booster vaccination (BM1). BM1 sampling was also defined as 14–56 days after booster vaccination. We had intended to sample at 1, 3, and 6 months after the third vaccination (BM1, BM3, and BM6). However, the fourth vaccination wave started earlier than expected, meaning that some patients had received their fourth vaccination before BM3 sampling, while other patients expressed their wish not to receive a fourth. Because the number of available BM3 samplings was expected to be too small, we canceled the scheduled samplings after BM1. Written informed consent was obtained from all participants. Methods were conducted in compliance with the Declaration of Helsinki and the relevant guidelines. The study was approved by the Institutional Review Board of Fukuoka University Hospital (approval number: H21-263 and H22-01–009) and Hara-Sanshin Hospital (approval number: 2021–06).
Measurement of anti-SARS-CoV-2 antibody
The detailed protocol for the enzyme-linked immunosorbent assay used in this study was previously reported [12]. In short, we used recombinant protein of the SARS-CoV-2 S-protein receptor-binding domain, which was expressed and purified based on a previously reported method [13], as antigen, 200-fold diluted serum samples as the primary antibody, and horseradish peroxidase-labeled goat anti-human IgG antibody as the secondary antibody. Following addition of 3,3′5,5′-tetramethylbenzidine substrate, absorbance at 450 nm was measured, and the absorbance of the blank well was subtracted to determine absorbance 450 (ABS450). The cutoff value of ABS450 for determining the presence of infection was 0.348, with 100% sensitivity and 99% specificity [12]. For further quantitative analysis, we performed six fourfold stepwise dilutions starting at 200-fold and determined the monoclonal antibody equivalent by measuring samples and drawing a calibration curve using human anti-SARS-CoV-2S1 monoclonal IgG. The monoclonal antibody equivalent (ng/ml) divided by 200 was defined as arbitrary units (AU) and used for analysis. The following reagents were obtained through BEI Resources, NIAID, NIH: vector pCAGGS containing the SARS-related coronavirus 2, Wuhan-Hu-1 spike glycoprotein RBD, NR-52309 (produced under HHSN272201400008C).
Evaluation of the doses of immunosuppressive agents and additional analyses
Tacrolimus and ciclosporin A doses were adjusted using trough levels. Regarding mycophenolate mofetil (MMF), both institutes currently have a fixed dose policy and do not perform therapeutic drug monitoring. Thus, we evaluated the daily dose per body weight (mg/kg/day) and used this in the subsequent analyses. Serial changes in immunosuppression were evaluated by the correlations between post-transplant years and MMF dose and tacrolimus trough level. We also compared MMF dose and tacrolimus trough levels between male and female patients, and between those with ABO-compatible (ABO-C) KT and ABO-I KT. In addition, we investigated the effect of heterologous boost (switched from BNT162b2 to mRNA-1273 or vice versa at third vaccination) in members of the KT group who had failed to develop antibodies after the second vaccination.
Statistical analysis
Data are expressed as mean ± standard deviation (SD), median, and interquartile range (IQR), as appropriate. Chi-squared test was used to compare categorical data, and Mann–Whitney U test or unpaired t test was used to compare variables between groups. To evaluate serial changes in antibody levels, analysis of variance was used. Univariable and multivariable logistic regression analyses were performed to identify factors associated with antibody acquisition at M1. Age, sex, and variables showing P values < 0.1 in univariable analysis were used for the multivariable analysis. Because rituximab was used in all ABO-incompatible (ABO-I) ABO-I KT patients, ABO-I KT was used as a variable. We also included BMI because another Japanese study revealed that high BMI was associated with better antibody response [14]. Results were expressed as odds ratios (ORs) with 95% confidence intervals (CI). Statistical analyses were conducted using GraphPad Prism (GraphPad Software, San Diego, CA, USA) and JMP version 14.2.0 (SAS Institute, Cary, NC, USA). A two-tailed P value of < 0.05 was considered significant.
Results
Baseline characteristics of KT patients and controls
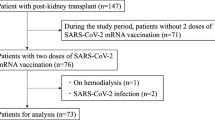
One hundred eighteen KT patients agreed to participate in the study. Of these, 13 who underwent vaccination or developed COVID-19 before baseline sampling or who did not receive vaccination were excluded. We investigated 105 KT patients with at least two vaccine doses. Mean age, female sex, and BMI of 105 KT patients and 57 controls were 49.0 ± 12.0 vs 49.5 ± 11.1 years old, 40 (37.7%) vs 21 (36.8%), and 22.4 ± 5.0 vs 21.9 ± 3.5 kg/m2, respectively. Other clinical characteristics of the KT patients are summarized in Table 1. Regarding immunosuppressive therapy, all patients received basiliximab induction followed by a triple drug regimen mainly consisting of tacrolimus, MMF, and steroids. Rituximab (200 mg/body, single dose) was administered for ABO-I KT or patients having donor-specific antibody (DSA). Ciclosporin A was used instead of tacrolimus, and MMF was switched to azathioprine or everolimus in some patients because of side effects or episodic infection after KT.
Antibody acquisition after second vaccination
Some samples were obtained outside the scheduled period because of changes of scheduled visits by patients. We excluded these samples from the analysis: there were 98 (92.4%) M1 samples, 88 (83.0%) M3, and 85 (80.2%) M6. Among 87 KT patients who received the third vaccination, we obtained 65 samples (74.7%) during the BM1 sampling period (Supplemental Fig. 1).
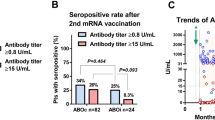
Antibody levels indicated by OD450 at baseline and M1 in the KT and control groups are shown in Fig. 1A. At M1, the antibody acquisition rate in the controls was 100% and only 36.7% in KT patients (P < 0.001). Median (IQR) antibody levels were 106.4 (79.7–167.6) AU in the controls and 4.9 (2.1–27.7) AU (P < 0.001) in antibody-positive KT patients. Serial changes in antibody levels until M6 are shown in Fig. 1B. All controls maintained positivity until M6. However, median antibody levels were decreased from 106.4 AU to 35.3 AU at M3, and 17.7 AU at M6 (P < 0.001). In KT patients, changes in antibody acquisition rates (36.7%, 39.3%, and 34.7%, respectively) and antibody-positive patients (4.9, 3.9, and 5.9 AU, respectively) were unremarkable.
Factors associated with antibody acquisition in KT group
Clinical characteristics of KT patients showing antibody positivity and negativity at M1 are summarized in Table 2. The antibody-positive group tended to have fewer female patients, longer post-transplant periods, and fewer ABO-I KT patients. Rituximab and MMF use was significantly lower in the antibody-positive group. Distributions of antibody levels at M1 by sex are shown in Fig. 2A: antibody levels among positive patients were 3.48 (0.96–23.27) in males and 1.42 (0.92–2.11) in females (P = 0.13). Associations between antibody levels and time since KT are shown in Fig. 2B. A weak but significant correlation was observed between time since KT and antibody levels. Antibody measurement at M1 revealed low antibody levels in those with ABO-I KT and MMF use (Fig. 2C, D). Because the supply of mRNA-1273 was quite low during the first two vaccination periods in Japan, we could not compare the effects of the two vaccines. Univariable and multivariable logistic regression analyses to identify factors associated with antibody acquisition at M1 are shown in Table 3. Time since KT (OR 1.107, 95% CI 1.012–1.211, P = 0.017), ABO-I KT (OR 0.316, 95% CI 0.101–0.991, P = 0.037), and MMF use (OR 0.177, 95% CI 0.054–0.570, P = 0.003) were significant predictors for antibody acquisition.
Antibody levels in KT group by different clinical characteristics. A Antibody levels at M0 and M1 in male and female KT patients. B Correlation between antibody levels and time since KT (years). C Antibody levels at M0 and M1 in KT patients with and without rituximab for desensitization. D Antibody levels at M0 and M1 in KT patients with and without MMF for maintenance immunosuppression
Dose of immunosuppressive agents over time and between subgroups
We investigated serial changes in MMF dose and found a significantly negative correlation with years since KT (Supplemental Fig. 2), while the correlation between tacrolimus trough level and years since KT was not significant. Female patients tended to have a lower antibody response (Table 2 and Fig. 2A). We investigated MMF dose by sex, and identified significantly higher doses in females than males (18.2 ± 5.8 vs 14.5 ± 3.4 mg/kg/day, P = 0.0006; Supplemental Fig. 2B). Furthermore, we also compared maintenance immunosuppression between ABO-I and ABO-C KT patients, and found that MMF doses were comparable (15.8 ± 4.4 vs 16.3 ± 5.3 mg/kg/day, P = 0.70; Supplemental Fig. 2C), but in the patients treated with tacrolimus, the trough level was higher in those with ABO-I KT than in those with ABO-C KT (5.6 ± 0.9 vs 4.7 ± 1.5 ng/mL, P = 0.005; Supplemental Fig. 2D).
Antibody acquisition after third vaccination
Eighty-seven KT patients received a third vaccination, but BM1 sampling could not be performed in 65 of these. The antibody acquisition rate in the controls was maintained at 100% at BM1. In the KT group, the antibody acquisition rate increased to 75.3%. Median antibody levels in the controls were significantly increased from 17.7 (10.7–26.9) AU at M6 to 284.5 (198.1–536.0) AU at BM1 (P < 0.001). KT patients also showed increased antibody levels from 5.9 (2.8–11.7) AU to 71.7 (31.9–167.4) AU (P < 0.001). Figure 3A indicates the distribution of antibody levels in the KT and control groups at M1 and BM1. Notably, in the KT group, antibody levels were widely distributed at M1, whereas the distribution can be divided into responders and non-responders at BM1. Distribution of antibody levels at M1 and BM1 in KT patients by sex is shown in Fig. 3B; there seemed to be no sex differences at BM1.
Antibody levels after third vaccination in KT and control groups, and different clinical characteristics in KT group. A Comparison between M1 and 1 month after the third vaccination (BM1) in KT and control groups. B Antibody levels at M1 and BM1 in male and female KT patients. C. Antibody levels at M1 and BM1 among patients who failed to develop antibodies after the first two vaccinations by homologous or heterologous boost
Among 65 KT patients with BM1 sampling, 32 showed antibody positivity before third vaccination. The remaining 33 patients failed to develop antibodies after the first two vaccine doses. Of these, 17 patients (51.5%) showed de novo antibody acquisition and 16 (48.5%) remained negative. Clinical characteristics between de novo antibody-positive and -negative KT patients are shown in Table 4. We could not identify factors that differed between the de novo antibody-positive and -negative subgroups. The effect of heterologous booster vaccinations is currently of interest because it may induce comparable or higher antibody titers [14]. Changes in antibody levels between homologous and heterologous booster subgroups were investigated and suggested no significant effect (Fig. 3C).
Clinical outcomes
Among 105 KT patients, 4 developed COVID-19 between the second and third vaccines, and 2 patients without third vaccination developed COVID-19 later than M6 sampling (Supplemental Fig. 1). Antibody analysis prior to COVID-19 development was negative in these six patients. However, 8 of 87 patients with third vaccination developed COVID-19, and all were antibody-positive until M6, so-called breakthrough infection. One patient with COVID-19 died of fatal arrhythmia rather than pneumonia or multiple organ failure. No KT patient developed acute rejection nor graft loss during the study period. Only two controls developed COVID-19 after third vaccination. The third vaccination period corresponded to Omicron BA.5 variant prevalence in Japan, and many breakthrough infection cases were reported among the general population.
Discussion
We demonstrated that antibody acquisition rates and antibody levels were low after two SARS-CoV-2 vaccinations in Japanese KT patients, consistent with previous reports from other countries and Japanese institutes [10, 15,16,17,18,19]. In our study, factors associated with antibody acquisition after the second vaccination were time since KT, ABO-I KT, and MMF use. Continuous measurement revealed that the third vaccination increased both antibody acquisition rates and antibody levels, and no factors for de novo antibody response were identified. At the time of the first two vaccinations, BNT162b2 was disproportionately being distributed in Japan because the supply of mRNA-1273 was still low, and we could not compare the effects of the two vaccines. At the third vaccination, more patients received mRNA-1273. However, we did not identify an effect of heterologous boosters.
Many studies have investigated the antibody response in KT, and factors associated with antibody acquisition differed across the studies, which could be caused by assays, immunosuppressive protocol, and patient demographics. However, immunosuppressive therapy could be the most important determinant of antibody acquisition, which could explain most of our results. First, as in other studies, MMF rather than other agents was a significant factor for negative antibody responses. MMF is a potent antimetabolite that widely suppresses immunocompetent cells. A previous study demonstrated a dose-dependent relationship between MMF use and SARS-CoV-2 antibody level [20]. In addition, it has been reported that the antibody response was improved by temporary reduction or discontinuation of MMF at the time of vaccination [16, 17]. Conversely, everolimus-based regimens are suggested to show high antibody acquisition rates [21]. In our study, more patients were treated with everolimus than MMF. Thus, a lower antibody response to MMF was more clearly detected. The relatively lower antibody response in female KT patients was inconsistent with our previous result demonstrating that healthy male subjects showed a worse antibody response than women [12]. We observed a higher MMF dose per body weight in female patients, suggesting that differences in body weight might affect the strength of immunosuppression by MMF. The post-transplantation period was also identified as a predictor of antibody response, and an additional analysis revealed a negative correlation between MMF dose per body weight and years since KT (Supplemental Fig. 2A), suggesting that the better antibody response in patients with a longer post-transplant period might be caused by the reduction in maintenance MMF dose.
ABO-I KT was also identified as a factor for negative antibody response. ABO-I KT is performed in Japan to expand living donor indication. Because ABO-I KT is uncommon in other countries, there are few studies that include ABO-I KT as a variable, and all of them are from Japan [17, 18, 23]. These studies do not discuss the causal relationship between ABO-I KT and antibody response in detail. Therefore, we discuss rituximab as pre-transplant desensitization and maintenance immunosuppressive drugs separately. Several studies of KT demonstrated that rituximab was associated with poor antibody response [18, 19, 22], whereas others suggested no association [17, 23, 24]. There are several meta-analyses of antibody response in solid organ transplantation, but none analyzed the effect of rituximab [25]. Other studies focusing on rheumatic diseases suggested that the number of rituximab courses and cumulative dose are associated with antibody response [26], and antibody levels gradually improve over time until 9 months after the last rituximab administration [27]. In our study, single- and low-dose rituximab was used for desensitization, and time since KT ranged 1–30 years. Rituximab is unlikely to affect antibody response in most of these patients, and the presence of confounders is suggested. The clinical background of ABO-C and ABO-I KT (Supplemental Table) indicated that most parameters were comparable between the two groups, but diabetic nephropathy was common in ABO-I KT (43.3% vs 21.3%, P = 0.03), and frequency of positive DSA tended to be high (10.0% vs 1.3%, P = 0.07). Regarding maintenance of immunosuppression, MMF dose per body weight was comparable, but in the tacrolimus subgroup, trough level was higher in ABO-I KT (Supplemental Fig. 2C, D). These findings suggest that antibody response could be affected by rituximab in the early period, but higher tacrolimus level is important in the late period in ABO-I KT.
The strength of this study is that it is a prospective investigation in Japanese patients. Many participants received third vaccinations, and we confirmed a booster effect. Furthermore, we investigated factors associated with antibody acquisition in detail. There are also limitations of this study. First, the sample size was small, which could reduce statistical power. It is difficult to incorporate more participants because most Japanese citizens have already received at least two doses of a COVID-19 vaccine. Second, the antibody levels observed in this study cannot be compared with those of other studies because in-house assays were used. Third, we could not estimate the preventive effect of the antibody response because a variant subsequent to the original targeted strain caused many breakthrough infections. Fourth, we did not estimate cellular immunity, which is also an important factor. Despite these limitations, this study provides important information about the management of KT patients including anti-infection measures and booster vaccinations.
In conclusion, antibody response after two SARS-CoV-2 vaccinations was low in Japanese KT patients. Time since KT, ABO-I KT, and MMF for maintenance all affect antibody acquisition, and the mechanisms can be explained mostly by immunosuppressive therapy. Although antibody status improved after the third vaccination, many patients developed breakthrough infection. Repeated vaccinations and anti-infection measures are important until further attenuation of the virus and resolution of the pandemic.
Data Availability
Data in this study is not available because study protocol does not include the provision of patients’ information and samples to other facilities.
References
Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39:2791–9.
Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73:2065–72.
Terpos E, Trougakos IP, Apostolakou F, et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021;96:E257–9.
Bayart JL, Morimont L, Closset M, et al. Confounding factors influencing the kinetics and magnitude of serological response following administration of BNT162b2. Microorganisms. 2021;9:1340.
Kageyama T, Ikeda K, Tanaka S, et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 1861;2021(27):e1-5.
Chung EYM, Palmer SC, Natale P, et al. Incidence and outcomes of COVID-19 in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2021;78:804–15.
Grupper A, Sharon N, Finn T, et al. Humoral response of the Pfizer BNT 162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16:1037–42.
Hou YC, Lu KC, Kuo KL. The efficacy of COVID-19 vaccines in chronic kidney disease and kidney transplantation patients: a narrative review. Vaccines. 2021;9:885.
Danthu C, Hantz S, Dahlem A, et al. Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32:2153–8.
Caillard S, Thaunat O. COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol. 2021;17:785–7.
Okumi M, Toki D, Nozaki T, et al. ABO-incompatible living kidney transplants: evolution of outcomes and immunosuppressive management. Am J Transplant. 2016;16:886–96.
Sakamoto A, Yoshimura M, Itoh R, et al. Longitudinal dynamics of SARS-CoV-2 IgG antibody responses after the two-dose regimen of BNT162b2 vaccination and the effect of a third dose on healthcare workers in Japan. Vaccines. 2022;10:830.
Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033.
Garg I, Sheikh AB, Pal S, Shekhar R. Mix-and-match COVID-19 vaccinations (heterologous boost): a review. Infect Dis Rep. 2022;14:537–46.
Fujieda K, Tanaka A, Kikuchi R, et al. Antibody response to double SARS-CoV-2 mRNA vaccination in Japanese kidney transplant recipients. Sci Rep. 2022;12:6850.
Osmanodja B, Ronicke S, Budde K, et al. Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. J Clin Med. 2022;11:2565.
Miura M, Fukumoto M, Komatsu N, Shunto R, Harada H, Sasaki H. Temporary reduction of immunosuppression enhances production of anti-S antibody against severe acute respiratory syndrome coronavirus 2 after vaccination in kidney transplant recipients. Int J Urol. 2022, Online ahead of print.
Ishida H, Furusawa M, Unagami K, Omoto K, Iizuka J, Takagi T. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a retrospective cohort study at a single transplant institute in Japan. Exp Clin Transplant. 2022;5:463–71.
Hayama T, Hatakeyama S, Yoneyama T, et al. Seroprevalence of SARS-CoV-2 spike IgG antibodies after the second BNT162b2 mRNA vaccine in Japanese kidney transplant recipients. Sci Rep. 2022;12:5876.
Kantauskaite M, Müller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22:634–9.
de Boer SE, Berger SP, van Leer-Buter CC, et al. Enhanced humoral immune response after COVID-19 vaccination in elderly kidney transplant recipients on everolimus versus mycophenolate mofetil-containing immunosuppressive regimens. Transplantation. 2022;106:1615–21.
Haskin O, Ashkenazi-Hoffnung L, Ziv N, et al. Serological response to the BNT162b2 COVID-19 mRNA vaccine in adolescent and young adult kidney transplant recipients. Transplantation. 2021;105:e226–33.
Takai S, Nishida H, Ito H et al. Immunogenicity and safety of two doses of SARS-CoV-2 mRNA vaccine in kidney transplant recipients with low-dose rituximab. Int J Urol. 2022, Online ahead of print.
Ohki Y, Kawabe M, Yamamoto I, et al. Long-term humoral response after a second dose of SARS-CoV-2 mRNA vaccine in Japanese kidney transplant recipients. Front Microbiol. 2022;13: 922042.
Zong K, Peng D, Yang H, et al. Risk factors for weak antibody response of SARS-CoV-2 vaccine in adult solid organ transplant recipients: a systemic review and meta-analysis. Front Immunol. 2022;13: 888385.
Furer V, Eviatar T, Zisman D, et al. Predictors of immunogenic response to the BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases treated with rituximab. Vaccines. 2022;10:901.
van der Togt CJT, Ten Cate DF, den Broeder N, Rahamat-Langendoen J, van den Bemt BJF, den Broeder AA. Humoral response to coronavirus disease-19 vaccines is dependent on dosage and timing of rituximab in patients with rheumatoid arthritis. Rheumatology 2022;61: SI175–9.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JP21K16329 to M.Y.). The authors thank H. Nikki March, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests
Ethical approval
The protocol for this research project was approved by the ethics committees of Fukuoka University Hospital (approval number: H21-263 and H22-01–009) and Hara Sanshin Hospital (approval number: 2021–06) and it conformed to the provisions of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all of the subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Table 1. Clinical background of ABO-C and ABO-I KT in the studied patients (DOCX 21 KB)
10157_2023_2334_MOESM2_ESM.pptx
Supplemental Figure 1. Flow chart showing the process of patient registration, blood sampling, and development of COVID-19 (PPTX 46 KB)
10157_2023_2334_MOESM3_ESM.pptx
Supplemental Figure 2. MMF dose per body weight at baseline and clinical characteristics. A. Correlation between time since KT and MMF dose per body weight. B. MMF dose per body weight in male and female KT patients. C: MMF dose per body weight in ABO-C and ABO-I KT. D. Tacrolimus trough levels in ABO-C and ABO-I KT (PPTX 81 KB)
About this article
Cite this article
Deguchi, H., Sakamoto, A., Nakamura, N. et al. Antibody acquisition after second and third SARS-CoV-2 vaccinations in Japanese kidney transplant patients: a prospective study. Clin Exp Nephrol 27, 574–582 (2023). https://doi.org/10.1007/s10157-023-02334-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-023-02334-0