Abstract
Background
High-risk screening for Fabry disease in dialysis patients is an effective means for reducing the number of undiagnosed cases. However, such screening has not been conducted in Chiba Prefecture, Japan. Herein, we aimed to estimate the prevalence of Fabry disease among patients undergoing hemodialysis in Chiba Prefecture by high-risk screening using α-galactosidase A (αGal A) activity measurement, and examine the hemodialysis effect on αGal A activity.
Methods
Patients who underwent maintenance hemodialysis at 25 facilities in Chiba Prefecture were recruited. The αGal A activity was measured using the dried blood spot (DBS) test as the first screening. If the enzyme activity was lower than the cut-off, the second screening was performed with the same method before and after dialysis.
Results
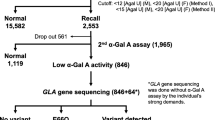
Overall, 2924 patients (2036 men and 888 women) were included from which 94 cases (45 men and 48 women) showed decreased αGAL activity in the first screening and 3 (two men and one women) in the second screening. Genetic testing was performed in 3 patients, and the c.1078G > A mutation in GLA gene was detected in one male patient (0.03%). There has been a statistically significant decrease in αGal A activity of DBS at post-dialysis compared to that at pre-dialysis (20.5 ± 10.4 pmol/h/disk and 22.7 ± 11.5 pmol/h/disk, p < 0.0001).
Conclusion
The prevalence of Fabry disease among patients undergoing hemodialysis in Chiba Prefecture was estimated as 0.03%. This is the first time that dialysis has been shown to affect the αGal A activity.


Similar content being viewed by others
References
Desnick RJ, Ioannou YA, Eng CM. Alpha-galactosidase a deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. p. 3733–74.
Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease: Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–7.
Feriozzi S, Rozenfeld P. Pathology and pathogenic pathways in Fabry nephropathy. Clin Exp Nephrol. 2021;25:925–34.
Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ. International collaborative Fabry disease study group Fabry disease study group. safety and efficacy of recombinant human alpha-galactosidase a replacement therapy in Fabry’s disease. N Engl J Med. 2001;345:9–16.
Germain DP, Hughes DA, Nicholls K, Bichet DG, Giugliani R, Wilcox WR, Feliciani C, Shankar SP, Ezgu F, Amartino H, Bratkovic D, Feldt-Rasmussen U, Nedd K, Sharaf El Din U, Lourenco CM, Banikazemi M, Charrow J, Dasouki M, Finegold D, Giraldo P, Goker-Alpan O, Longo N, Scott CR, Torra R, Tuffaha A, Jovanovic A, Waldek S, Packman S, Ludington E, Viereck C, Kirk J, Yu J, Benjamin ER, Johnson F, Lockhart DJ, Skuban N, Castelli J, Barth J, Barlow C, Schiffmann R. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med. 2016;375:545–55.
Ortiz A, Kanters S, Hamed A, DasMahapatra P, Poggio E, Maski M, Aguiar M, Ponce E, Jansen JP, Ayers D, Goldgrub R, Desnick RJ. Agalsidase beta treatment slows estimated glomerular filtration rate loss in classic Fabry disease patients: results from an individual patient data meta-analysis. Clin Kidney J. 2020;14:1136–46.
Marchesoni CL, Roa N, Pardal AM, Neumann P, Cáceres G, Martínez P, Kisinovsky I, Bianchi S, Tarabuso AL, Reisin RC. Misdiagnosis in Fabry disease. J Pediatr. 2010;156:828–31.
Yoshida S, Kido J, Sawada T, Momosaki K, Sugawara K, Matsumoto S, Endo F, Nakamura K. Fabry disease screening in high-risk populations in Japan: a nationwide study. Orphanet J Rare Dis. 2020;15:220.
Wasserstein MP, Orsini JJ, Goldenberg A, Caggana M, Levy PA, Breilyn M, Gelb MH. The future of newborn screening for lysosomal disorders. Neurosci Lett. 2021;760:136080.
Nakagawa N, Sawada J, Sakamoto N, Takeuchi T, Takahashi F, Maruyama JI, Momosaki K, Nakamura K, Endo F, Hasebe N. High-risk screening for Anderson–Fabry disease in patients with cardiac, renal, or neurological manifestations. J Hum Genet. 2019;64:891–8.
Sawada T, Kido J, Sugawara K, Matsumoto S, Takada F, Tsuboi K, Ohtake A, Endo F, Nakamura K. Detection of novel Fabry disease-associated pathogenic variants in Japanese patients by new born and high-risk screening. Mol Genet Genomic Med. 2020;8: e1502.
Sawada T, Kido J, Sugawara K, Nakamura K. High-risk screening for Fabry disease: a nationwide study in Japan and literature review. Diagnostics (Basel). 2021;11:1779.
West M, Nicholls K, Mehta A, Clarke JT, Steiner R, Beck M, Barshop BA, Rhead W, Mensah R, Ries M, Schiffmann R. Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol. 2009;20:1132–9.
Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, Yoshida A, Kanzaki T, Enriquez AL, Eng CM, Tanaka H, Tei C, Desnick RJ. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int. 2003;64:801–7.
Azpiazu D, González-Parra E, Egido J, Villa-Bellosta R. Hydrolysis of extracellular pyrophosphate increases in post-hemodialysis plasma. Sci Rep. 2018;8:11089.
Schulz AM, Terne C, Jankowski V, Cohen G, Schaefer M, Boehringer F, Tepel M, Kunkel D, Zidek W, Jankowski J, European Uremic Toxin Work Group (EUTox). Modulation of NADPH oxidase activity by known uraemic retention solutes. Eur J Clin Invest. 2014;44:802–11.
Maruyama H, Miyata K, Mikame M, Taguchi A, Guili C, Shimura M, Murayama K, Inoue T, Yamamoto S, Sugimura K, Tamita K, Kawasaki T, Kajihara J, Onishi A, Sugiyama H, Sakai T, Murata I, Oda T, Toyoda S, Hanawa K, Fujimura T, Ura S, Matsumura M, Takano H, Yamashita S, Matsukura G, Tazawa R, Shiga T, Ebato M, Satoh H, Ishii S. Effectiveness of plasma lyso-Gb3 as a biomarker for selecting high-risk patients with Fabry disease from multispecialty clinics for genetic analysis. Genet Med. 2019;21:44–52.
Dobrovolny R, Dvorakova L, Ledvinova J, Magage S, Bultas J, Lubanda JC, Elleder M, Karetova D, Pavlikova M, Hrebicek M. Relationship between X-inactivation and clinical involvement in Fabry heterozygotes. Eleven novel mutations in the alpha-galactosidase a gene in the Czech and Slovak population. J Mol Med (Berl). 2005;83(8):647–54.
Manabe S, Mochizuki T, Sato M, Kataoka H, Taneda S, Honda K, Uchida K, Nitta K. Lupus nephritis and hydroxychloroquine-associated zebra bodies: not just in Fabry disease. Kidney Med. 2021;3:442–6.
Tsukimura T, Shiga T, Saito K, Ogawa Y, Sakuraba H, Togawa T. Does administration of hydroxychloroquine/amiodarone accelerate accumulation of globotriaosylceramide and globotriaosylsphingosine in Fabry mice? Mol Genet Metab Rep. 2021;28:100773.
Shimohata H, Miyake Y, Yoshida Y, Usui J, Mori T, Sohara E, Uchida S, Hirayama K, Kobayashi M. LMX1B-associated nephropathy that showed myelin figures on electron microscopy. CEN Case Rep. 2021;10:588–91.
Sakuraba H, Tsukimura T, Tanaka T, Togawa T, Takahashi N, Mikami D, Wakai S, Akai Y. Clinical and biochemical investigation of male patients exhibiting membranous cytoplasmic bodies in biopsied kidney tissues; a pitfall in diagnosis of Fabry disease. J Nephropathol. 2015;4:91–6.
Nitta K, Goto S, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, Nakai S, Wada A, Hamano T, Hoshino J, Joki N. Annual dialysis data report for 2018, JSDT renal data registry: survey methods, facility data, incidence, prevalence, and mortality. Ren Replace Ther. 2020;6:41.
Nagata A, Nasu M, Kaida Y, Nakayama Y, Kurokawa Y, Nakamura N, Shibata R, Hazama T, Tsukimura T, Togawa T, Saito S, Sakuraba H, Fukami K. Screening of Fabry disease in patients with chronic kidney disease in Japan. Nephrol Dial Transplant. 2021;37:115–25.
Acknowledgements
We would like to express our deepest gratitude to the staff of the medical institutions who helped in recruiting patients to participate in this study, and to the responsible doctors at those respective institutions (Supplemental Summary S1). We would like to thank Akiko Sawada, Mori Tachibana and Keiko Tanaka for their administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Research involving human participants and/or animals
This study was conducted in accordance with the Declaration of Helsinki and with the approval by the ethics committee and institutional review board of Chiba-Higashi National Hospital (No. R1-13) and each hospital participated in this study.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Imasawa, T., Murayama, K., Sawada, T. et al. High-risk screening for Fabry disease in hemodialysis patients in Chiba Prefecture, Japan. Clin Exp Nephrol 27, 288–294 (2023). https://doi.org/10.1007/s10157-022-02295-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02295-w