Abstract
Background
Coronary artery calcification (CAC) is predictive of cardiovascular events. We assessed whether a non-calcium-based phosphate binder, lanthanum carbonate (LC), could delay CAC progression compared with a calcium-based phosphate binder, calcium carbonate (CC), in hemodialysis patients.
Methods
This was a subsidiary of the LANDMARK study, which is a multicenter, open-label, randomized control study comparing LC and CC for cardiovascular events among Japanese hemodialysis patients with hyperphosphatemia who were at risk of vascular calcification. Participants were randomly assigned (1:1) to receive LC or CC. The changes in the total Agatston score of CAC 2 years from baseline were the primary outcome. Secondary outcomes included the changes in the total Agatston score at 1 year from baseline and the changes in serum phosphate, corrected calcium, and intact parathyroid hormone concentrations.
Results
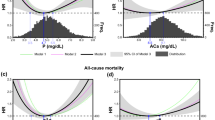
Of 239 patients, 123 comprised the full analysis set. The median daily drug dose (mg) was 750 [interquartile range (IQR), 750‒1500] in the LC group and 3000 (IQR, 3000‒3000) in the CC group; it remained constant throughout the study period. There was no significant difference in the change in total Agatston score from baseline to 2 years between the LC and CC groups [368 (95% confidence interval, 57–680) in the LC group vs. 611 (105–1118) in the CC group; difference, 243 (− 352–838)].
Conclusions
LC-based treatment for hyperphosphatemia did not delay CAC for 2 years compared with CC-based treatment in hemodialysis patients with at least one risk factor for vascular calcification.




Similar content being viewed by others
References
Goodman WG. Vascular calcification in end-stage renal disease. J Nephrol. 2002;15(Suppl 6):S82–5.
Goodman WG, London G, Amann K, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–9.
London GM, Marchais SJ, Guérin AP, Métivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens. 2005;14:525–31.
Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–41.
Russo D, Corrao S, Battaglia Y, et al. Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int. 2011;80:112–8.
Floege J, Ketteler M. Vascular calcification in patients with end-stage renal disease. Nephrol Dial Transplant. 2004;19(Suppl 5):V59-66.
Yamada K, Fujimoto S, Nishiura R, et al. Risk factors of the progression of abdominal aortic calcification in patients on chronic haemodialysis. Nephrol Dial Transplant. 2007;22:2032–7.
Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–605.
Shroff R, Long DA, Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179–89.
Kidney disease: Improving global outcomes CKD-MBD Working Group. KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7:1–59.
Ruospo M, Palmer SC, Natale P, et al. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst Rev. 2018;8:CD006023.
Ogata H, Fukagawa M, Hirakata H, et al. Effect of treating hyperphosphatemia with lanthanum carbonate vs calcium carbonate on cardiovascular events in patients with chronic kidney disease undergoing hemodialysis: the LANDMARK randomized clinical trial. JAMA. 2021;325:1946–54.
Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–36.
Karohl C, D’Marco Gascón L, Raggi P. Noninvasive imaging for assessment of calcification in chronic kidney disease. Nat Rev Nephrol. 2011;7:567–77.
Ogata H, Fukagawa M, Hirakata H, et al. Design and baseline characteristics of the LANDMARK study. Clin Exp Nephrol. 2017;21:531–7.
Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–88.
Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
Bellasi A, Kooienga L, Block GA, et al. How long is the warranty period for nil or low coronary artery calcium in patients new to hemodialysis? J Nephrol. 2009;22:255–62.
Treat to Goal Working Group, Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–52.
Qunibi W, Moustafa M, Muenz LR, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the calcium acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–65.
Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–24.
Kakuta T, Tanaka R, Hyodo T, et al. Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis. 2011;57:422–31.
Patel L, Bernard LM, Elder GJ. Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2016;11:232–44.
Toida T, Fukudome K, Fujimoto S, et al. Effect of lanthanum carbonate vs. calcium carbonate on serum calcium in hemodialysis patients: a crossover study. Clin Nephrol. 2012;78:216–23.
Zhang C, Wang S, Zhao S, Zhang X. Effect of lanthanum carbonate on coronary artery calcification and bone mineral density in maintenance hemodialysis patients with diabetes complicated with adynamic bone disease: a prospective pilot study. Medicine. 2017;96:e8664.
Fujii H, Kono K, Nakai K, et al. Effects of lanthanum carbonate on coronary artery calcification and cardiac abnormalities after initiating hemodialysis. Calcif Tissue Int. 2018;102:310–20.
Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG. Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: a pilot randomized controlled trial. Nephrology. 2011;16:290–8.
Wada K, Wada Y. Evaluation of aortic calcification with lanthanum carbonate vs. calcium-based phosphate binders in maintenance hemodialysis patients with type 2 diabetes mellitus: an open-label randomized controlled trial. Ther Apher Dial. 2014;18:353–60.
Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–39.
Dialysis Clinical Outcomes Revisited Investigators, Suki WN. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients: Results of a randomized clinical trial. J Ren Nutr. 2008;18:91–8.
Stirnadel-Farrant HA, Karaboyas A, Cizman B, et al. Cardiovascular event rates among hemodialysis patients across geographical regions-A snapshot from the dialysis outcomes and practice patterns study (DOPPS). Kidney Int Rep. 2019;4:864–72.
Kumssa DB, Joy EJ, Ander EL, et al. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci Rep. 2015;5:10974.
Acknowledgements
We acknowledge the contribution of the LANDMARK Investigators and Committees.
Funding
This study was supported by Bayer Yakuhin, Ltd. Bayer Yakuhin, Ltd. had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. However, the company was kept informed of the progress of the study. There were no confidentiality agreements regarding the data.
Author information
Authors and Affiliations
Consortia
Contributions
Research idea and study design: MF, HH, TA; data acquisition: TK; data analysis/interpretation: HO, MF, HH, TK, TA; statistical analysis: HO, TK; drafting of this manuscript: HO, TK; supervision or mentorship: MF, HH, TA. Each author contributed toward important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Corresponding author
Ethics declarations
Conflict of interest
Dr Ogata reported receiving lecture fees from Bayer Yakuhin, Kyowa Kirin, Torii Pharmaceutical, Otsuka, Kissei Pharmaceutical, Mitsubishi Tanabe Pharma, Sumitomo Dainippon Pharm, Daiichi Sankyo, Kowa, Ono Pharmaceutical; grants from Torii Pharmaceutical and Ono Pharmaceutical; and consulting fees from YL Biologics. Dr Fukagawa reported receiving personal fees from Bayer Yakuhin and grants from Kyowa Kirin. Dr Hirakata reported receiving personal fees from Kyowa-Kirin, Chugai Pharma, Torii, Japan Tobacco, and Ono Yakuhin. Dr Kagimura reported receiving grants from Bayer Yakuhin. Dr Akizawa reported receiving consulting and lecture fees from Bayer Yakuhin, Astellas, Kyowa Kirin, Kissei Pharmaceutical, Ono Pharmaceutical, Fuso Pharmaceutical Industry, Torii Pharmaceutical; consulting fees from GlaxoSmithKline, JT Pharmaceutical, Nipro Corporation, Otsuka, and Sanwa Chemical; and lecture fees from Chugai Pharmaceutical. No other disclosures were reported.
Ethical approval
The trial protocol of the LANDMARK study was previously published and was approved by the Institutional Review Board. All patients enrolled in this LANDMARK-SS study provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ogata, H., Fukagawa, M., Hirakata, H. et al. Effect of lanthanum carbonate and calcium carbonate on the progression of coronary artery calcification among hemodialysis patients with vascular calcification risk: a randomized controlled trial. Clin Exp Nephrol 26, 1223–1232 (2022). https://doi.org/10.1007/s10157-022-02270-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02270-5