Abstract
In recent years, large cohort studies of patients with chronic kidney disease (CKD) have been established all over the world. These studies have attempted to analyze the pathogenesis of CKD using a large body of published evidence. The design of cohort studies is characterized by the measurement of the exposure prior to the occurrence of the outcome, which has the advantage of clarifying the temporal relationship between predictors and outcomes and estimating the strength of the causal relationship between predictors and multiple outcomes. Recent advances in biostatistical analysis methods, such as propensity scores and risk prediction models, are facilitating causal inference using higher quality evidence with greater precision in observational studies. In this review, we will discuss clinical epidemiological research of kidney disease based on the analysis of observational cohort data sets, with a focus on our previous studies.
Similar content being viewed by others
Introduction
In general, epidemiology is defined as a scientific method for clarifying the relationship between disease events and the factors that influence them, and for establishing effective countermeasures against health-related problems. There are two types of epidemiological studies: observational studies, in which the relationship between factors and diseases is clarified by observation alone without artificial manipulation, and intervention studies, in which changes in the occurrence and prognosis of diseases before and after artificial manipulation are experimentally confirmed. It should be noted that the results obtained in observational studies cannot completely rule out the possibility of an apparent relationship arising owing to chance, bias, or confounding [1,2,3]. In cohort studies, the temporal relationship between the exposures and the onset of the disease event is clear, making it possible to infer causality. Therefore, the evidence level of cohort studies ranks higher than that of other study designs, such as case–control and cross-sectional studies.
Cohort studies can identify risk factors for disease by observing a population for a period and comparing differences in the incidence of disease events with and without exposure. Bias and confounding can be removed to a certain extent through statistical analysis, but a randomized controlled intervention trial (RCT) is required to completely remove the effects of bias and confounding. However, there are many barriers to the implementation of RCTs, such as cost and ethical concerns. Evidence from well-designed cohort studies should, therefore, form the basis for inferring interventional factors that are predicted to have a causal effect on disease. The pathogenesis of chronic kidney disease (CKD) involves a wide range of factors, including immunological and environmental factors and genetic predisposition, and these interact with each other. To clarify the prognostic factors of CKD, it is important to create a systematic database comprising the vast amount of clinical information generated in daily practice. By creating such a database, it will be possible to clarify factors that are unclear in individual cases alone and to elucidate the mechanisms of CKD onset and exacerbation. To extract high-quality evidence from observational databases that can be used in disease prevention and intervention studies, it is necessary to apply appropriate methods of statistical analysis that control confounding and lead to accurate estimates. In addition to the current status of CKD cohorts around the world, herein, we review biostatistical methods that are useful in clinical research to establish evidence that can be used to elucidate the pathogenesis of CKD and improve medical practice.
Representative non-dialysis-dependent CKD cohort studies
Cohort studies of non-dialysis-dependent CKD outside Japan
African American Study of Kidney Disease and Hypertension (AASK)
The AASK cohort study was a follow-up study of an RCT conducted among 1094 black patients with CKD, with three initial antihypertensive drugs (ramipril, metoprolol, and amlodipine) and two antihypertensive targets (mean blood pressure 102–107 mmHg and < 92 mmHg) [4, 5]. Participants comprised 691 consented patients out of 787 who did not develop end-stage kidney disease (ESKD) during the AASK study period and were followed for up to 6.4 years. The black population in the United States is at high risk for worsening kidney function, because they are more susceptible to hypertension and kidney impairment than Whites and have a higher incidence of lifestyle-related diseases, such as obesity. This follow-up study showed an association between apolipoprotein L1 (APOL1) gene polymorphism and worsening kidney function [6, 7]. Recently, metabolomic analysis using frozen serum samples has also shown the association of specific serum metabolites with urinary protein and total mortality [8, 9].
Kidney Early Evaluation Program (KEEP)
The KEEP study is focused on improving the prognosis of kidney disease by increasing public awareness about the concept of kidney disease and encouraging early medical intervention in high-risk patients. This study was originally initiated by the National Kidney Foundation in 2000 to raise awareness about CKD screening. The target population is the general population aged 18 years and older who have hypertension, diabetes, or a family history of hypertension, diabetes, or kidney disease. Screening tests that include items on social background, pre-existing medical conditions, blood pressure measurement, and blood and urine tests are conducted, and participants with abnormal values are encouraged to visit a medical institution. Follow-up data after medical care are not included but can be merged with external data such as the National Death Index, the United States Renal Data System, and the National Health and Nutrition Examination Survey (NHANES). By cross-checking with external data sources, the association between demographic and other data such as body mass index, racial differences, health insurance status, total mortality, dialysis initiation, and cardiovascular disease can be examined [10,11,12,13,14].
Chronic Renal Insufficiency Cohort (CRIC)
The CRIC study is a prospective cohort study initiated by the National Institute of Diabetes and Digestive and Kidney Diseases in 2001 to investigate risk factors for the progression of kidney disease and the development of CVD, and to gain useful knowledge for future intervention studies. This study has been ongoing for more than 20 years from Phase I to Phase IV in a total of 3612 patients with CKD attending seven renal care centers. In addition to the basic study, many ancillary studies have been conducted and many articles have been published in medical journals. Since its inception, the CRIC has been strategically collecting data related to the care of patients with CKD, and biospecimen storage has been thorough. Over the past decade, the CRIC study has contributed substantially to our understanding of factors associated with CKD progression. Hannan et al. summarized findings from a longitudinal study that assessed risk factors associated with CKD progression in the CRIC study under six themes, as follows: (1) socioeconomic factors (sex, race/ethnicity, nephrological care); (2) behavioral factors (healthy lifestyle, diet, sleep); (3) genetic factors (APOL1, genome-wide association studies [GWAS], renin–angiotensin–aldosterone pathway genes); (4) cardiovascular (atrial fibrillation, hypertension, vascular stiffness); (5) metabolic (fibroblast growth factor 23 and urinary oxalate); and (6) novel factors (biomarkers of acute kidney injury and kidney impairment) [15]. The current fourth phase, which began in 2018, is increasing the ethnic diversity of the cohort by recruiting 500 Native American and 126 Hispanic adults. In addition, data collection has been refocused on incorporating new mobile technologies to remotely collect kidney and cardiovascular data from participants’ homes, further highlighting the importance of interdisciplinary collaborative opportunities.
Chronic Kidney Disease in Children (CKiD)
The cohort was established in 2006 and has enrolled and prospectively followed more than 800 pediatric patients with CKD from 57 sites across the United States. Patients between the ages of 1 and 16 years with an estimated glomerular filtration rate (GFR) of 30–75 mL/min/1.73 m2, not including those receiving dialysis, were included in the study. In addition to examining risk factors for progression of kidney failure, the study is unique in that it addresses research questions specific to pediatric practice, such as the impact of kidney function on growth and neurological development, the relationship between stunting and mortality risk, and epidemiological studies of pediatric cardiac disease [16].
German Chronic Kidney Disease (GCKD) study
The GCKD study is a prospective, observational, nationwide cohort study established in 2010. The study aims to enroll 5000 patients with CKD of various etiologies undergoing nephrological treatment and to follow them for up to 10 years. Patient recruitment and follow-up are being organized by collaborating centers with nephrologists throughout the country through an academic nephrology network. Patients with an estimated GFR between 30 and 60 mL/min/1.73 m2 at the time of enrollment, or with overt proteinuria if GFR is > 60 mL/min/1.73 m2, are included. Biomaterials such as DNA, serum, plasma, and urine are collected using standardized methods to identify biomarkers associated with CVD incidence and CKD progression [17]. In addition to epidemiological topics such as blood pressure [18], heart failure [19], dietary patterns [20], and gout [21], biomarkers and genes such as urine 6-bromotryptophan [22], serum uromodulin [23], GWAS of urate and gout [24], telomere length [25], and other biomarkers and genes have been actively investigated.
Cohort studies of non-dialysis-dependent CKD in Japan
Chronic Kidney Disease Japan Cohort (CKD-JAC)
The CKD-JAC study was established in 2007 to determine the incidence of CVD, ESKD, and all-cause mortality in the Japanese population. The CKD-JAC is a 4-year prospective observational cohort of patients attending nephrology centers. Inclusion criteria are (1) Japanese and Asian patients living in Japan, (2) age 20–75 years, and (3) estimated GFR 10–59 mL/min/1.73 m2 [26]. To date, 15 studies have been published on CVD incidence, sleep, hospitalization events, and progression factors of left ventricular hypertrophy.
Gonryo study
The Gonryo study is a 5-year prospective cohort of 2692 outpatients with CKD from 11 centers that began in 2007. All patients met the criteria for CKD and had a persistently low GFR of less than 60 mL/min/1.73 m2 or proteinuria by urinalysis [27]. To date, six studies have reported on CVD incidence, anemia, blood pressure, and CKD progression.
Fukuoka Kidney disease Registry (FKR) study
The FKR is a prospective, multicenter, observational cohort of patients with CKD before dialysis that began enrollment in 2013. Approximately 4500 patients were enrolled in this study, with extensive lifestyle surveys and collection of biological samples including blood, urine, and DNA at baseline enrollment, followed by a planned 5-year follow-up, which is still underway [28].
The objectives of this cohort are as follows:
-
I.
To identify and validate biomarkers, including novel risk factors and genetic susceptibility, using biological materials and high-throughput omics technology;
-
II.
To clarify the effects of genetic and environmental interactions on the risk of adverse events in Japanese patients with CKD;
-
III.
To evaluate the impact of lifestyle issues such as sex, nutrition, exercise, quality of life (QOL), and psychological and socioeconomic factors on CKD progression and complications; and
-
IV.
To assess the socioeconomic burden of age-related complications and health resource utilization in older patients with CKD.
Together with the search for risk factors in epidemiological studies and multi-layered omics analysis, the findings of our cohort study will elucidate the molecular mechanisms that determine the phenotype of the disease and will contribute to personalized medicine and healthy longevity in patients with CKD.
Representative hemodialysis cohort studies
Hemodialysis cohort studies outside Japan
Dialysis Outcomes and Practice Patterns Study (DOPPS)
The DOPPS is a large, international observational study of practice patterns and patient outcomes in patients receiving maintenance hemodialysis. The data collected in each country will be sent for analysis to the Arbor Research Collaborative for Health, a non-profit research organization in the United States. The study sites will be randomly selected to be representative of dialysis facilities in each participating country, taking into account facility type and geographic distribution. The DOPPS is collecting data on four themes: (1) life expectancy, (2) hospitalization, (3) vascular access, and (4) QOL. It has been shown that mortality is largely explained by regional differences in survival (especially when comparing the United States and Europe) and by differences in the use of vascular access in facilities [29, 30]. The DOPPS also showed that longer treatment time was associated with lower mortality in models adjusted for Kt/V and other characteristics using both standard and instrumental variable analysis methods [31].
Hemodialysis cohort studies in Japan
Japan Dialysis Outcomes and Practice Patterns Study (J-DOPPS)
The J-DOPPS, a sub-cohort of the DOPPS, is a prospective observational study of Japanese patients receiving maintenance hemodialysis. J-DOPPS analysis has reported a better life expectancy for patients with maintenance hemodialysis in Japan than in the United States and Europe, and a higher use of arteriovenous fistulas in Japan [29, 30]. Recently, it has been shown that the rate of adherence to treatment plans (frequency and duration) and the number of physician visits are much higher in Japan than in Europe and the United States [32]. The wide range of new findings from the J-DOPPS will have a great impact on dialysis treatment in Japan and is expected to contribute to further improvement of patient outcomes.
Japanese Society for Dialysis Therapy Renal Data Registry (JRDR)
The JRDR is a database based on an annual survey conducted by the Japanese Society for Dialysis Therapy (JSDT), which has accumulated data on approximately 870,000 patients undergoing dialysis. In the past, reference values for guidelines have been formulated on the basis of the results of analyses of this database. In recent years, a number of important studies have emerged from this database that will have an important impact on dialysis care in Japan, including a study that showed an association between pre-dialysis serum magnesium concentration and total mortality, cardiovascular death, and non-cardiovascular death at 1 year [33], and a study that reported an association between hyponatremia and total mortality and cardiovascular risk [34].
Kyushu Prospective Cohort Study in Hemodialysis Patients (Q-Cohort Study)
We have conducted the Q-Cohort Study since 2006, a prospective cohort study of approximately 3600 patients on chronic hemodialysis, with outcomes including all-cause mortality, death from infection or tumor, major adverse cardiovascular events (MACE), and new fractures. The clinical background of included patients is very similar to that of Japanese patients undergoing dialysis as of December 31, 2006, as compiled and published by the JSDT, except for their longer dialysis time. To date, the following studies have been conducted: hypo-responsiveness to erythropoiesis-stimulating agents and increased risk of total mortality and MACE [35], use of vitamin D receptor agonists (VDRA) and decreased risk of infectious disease mortality [36], hyperphosphatemia and increased risk of cerebral hemorrhage [37], increased cardiothoracic ratio and increased risk of total mortality and MACE [38], muscle mass loss and increased risk of fracture [39], treatment-resistant hypertension [40], and multi-vascular disease and cardiovascular prognosis [41]. We are currently conducting a follow-up study to collect additional information such as cognitive function and frailty in a new target population by establishing a second population starting in 2019. The characteristics of the cohort studies described in this manuscript are shown in Table 1.
Biostatistical methods applicable to cohort data
To extract high-quality evidence from observational databases, it is necessary to apply appropriate methods of statistical analysis that control for confounding and lead to precise estimates. Currently, the search for novel disease risk markers is progressing, and the trend in clinical research is to create risk models that incorporate risk markers to stratify specific populations and test the efficacy of personalized treatment based on accurate individual risk prediction. In the following sections, we will outline the biostatistical methods that we have used and the evidence we have derived using these methods.
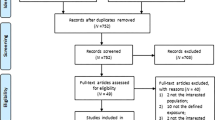
Risk prediction model
Clinical decisions regarding the diagnosis and treatment of individual patients are routinely determined under the constraint of various uncertainties. Therefore, obtaining accurate predictions of clinical prognosis is critically important for patient management of any disease. We developed and validated a clinical pathological prediction rule to calculate the absolute risk of long-term kidney prognosis in a large cohort of patients with immunoglobulin A (IgA) nephropathy [42]. The prediction rule can stratify patients according to the probability of future events by scoring clinical findings and the results of diagnostic tests. Since it is obvious that the internal validity of the prediction model generated by the derivation sample is high, the external validity of the model needs to be proved by testing the goodness of fit to another independent population (validation sample). Figure 1 shows brief summary of this study design. The prediction rule we created had nearly equivalent predictive performance for kidney prognosis in two independent cohorts (generation cohort, n = 698; validation cohort, n = 702), demonstrating its usefulness for stratifying the risk of developing ESKD using absolute risk in individual cases. Recently, Ueki et al. developed a risk model for the development of CVD consisting of risk factors such as age, history of CVD, diabetic nephropathy, history of dialysis, serum albumin concentration, and urinary protein at 1 year after transplantation in a cohort of kidney transplant recipients; the model showed good external validity in an independent external cohort [43]. Such prediction rules to guide initial treatment decisions will contribute to the realization of personalized medicine through validation in future prospective observational studies and clinical trials.
Risk reclassification
In recent years, the development of high-throughput measurement technologies has led to comprehensive exploratory studies of candidate compounds of interest in drug discovery and biomarker discovery research. The process of statistical validation is essential for the identification of new risk markers that can be applied to clinical practice. It is not enough for a novel risk marker to be an independent contributor to risk; it must also provide additional value in risk prediction over known and established risk markers. In other words, the relevance of a new risk marker should be assessed as an indicator of improved predictive performance of the risk model. The following criteria have been proposed for the evaluation phase of novel risk markers [44]: (i) discovery of novel marker; (ii) validation in a prospective cohort; (iii) confirmation of improved risk prediction; (iv) validation of clinical utility; (v) confirmation of improved clinical prognosis in interventional studies; and (vi) improvement in medical economy.
Receiver Operating Characteristic (ROC) curve, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) are frequently used as indices to evaluate the improvement of risk prediction ability of models in observational studies. The ROC curve is a two-dimensional graph that represents the discriminatory performance of a diagnostic test. The curve is drawn as a line plotting the true positive rate and false positive rate for each cutoff point that distinguishes abnormal from normal. The area under the ROC curve is called area under the curve (AUC), and an AUC value close to 1 means that the discriminatory ability of the diagnostic test is high. Reclassification is a statistical technique that examines how many patients can be reclassified by adding a new test or biomarker to an existing model for predicting the probability of the presence of a disease event. NRI can give clinical information about quantitative improvements by adding new biomarkers to traditional models. IDI can show that the addition of a new biomarker can improve sensitivity without sacrificing specificity. Therefore, the NRI and IDI are more sensitive than the AUC of ROC analysis, which shows improved predictive value [45, 46]. Figure 2 shows how Model 1 (existing model) is compared to Model 2 (new model) to calculate NRI and IDI values using the percentage of the population that has been reclassified.
Graphical representation of NRI (A) and IDI (B) for disease events. The NRI plot shows the proportion of individuals reclassified to higher or lower risk after the addition of biomarkers to the clinical model. The IDI plot shows the mean predicted probability of disease events according to the prior (Model1) and novel (Mode2) models
We investigated whether serum bilirubin could be a biomarker for the development of ESKD in an IgA nephropathy cohort [47]. In this study, we found that serum bilirubin is an independent risk factor for the development of ESKD, and the C-statistic of the model incorporating bilirubin level was improved compared with the conventional model using only risk factors. Evaluation using net reclassification improvement and integrated discrimination improvement also showed a significant improvement in risk discrimination. Such risk model evaluation emphasizes the value of serum bilirubin as a potential biomarker for predicting kidney prognosis in patients with IgA nephropathy.
Propensity score and instrumental variable methods
Covariate adjustment using the propensity score is useful to estimate the causal effect of treatment in observational studies where random assignment is not made and various types of confounding are likely to occur. The propensity score is the conditional probability of receiving the treatment rather than the control, given the observed covariates [48]. Specific adjustment methods using propensity scores include matching, stratification, analysis of covariance and inverse probability of treatment weighting (IPTW). The advantages and disadvantages of each statistical method are shown in Table 2. We used IPTW and instrumental variable methods to evaluate the effect of VDRA on infection-related mortality in a cohort of approximately 3500 hemodialysis patients (Q cohort study) [36]. We found a significant reduction in the risk of infection-related mortality in the intravenous group (n = 492) compared with the untreated group (n = 1007), and similarly, a significantly lower risk of mortality in the intravenous group compared with the oral group (n = 1878).
A limitation of the propensity score method is that the effects of unmeasured or unknown confounders cannot be adjusted. The best way to adjust for unmeasured confounders is to conduct RCTs; however, the ethical and cost burdens of conducting such studies make it difficult to implement in practice. Therefore, in recent years, the adjustment of unmeasured factors using the instrumental variable method has been attempted. An instrumental variable is a factor that is associated with the exposure but not with the outcome [49]. The objective of the instrumental variable, which affects the treatment group independently of the observed values, is to mimic the randomization of subjects into the treatment group. A schematic representation of the Instrumental variable method is shown in Fig. 3. In the study described above, we applied both propensity score analysis and instrumental variable methods to test the robustness of the analysis results. The results showed that the results differed depending on the incorporation of nutritional indicators into the model, suggesting that residual confounding related to nutrition may have affected the treatment effectiveness of VDRA.
System for promoting cohort studies
The most important points in promoting cohort studies are the establishment of a research organization and the development of human resources. The overall direction of the research analysis must be controlled under the supervision of the steering committee, which deliberates on the project plan, ethics, and validity of the study. The biostatistics team scientifically deliberates on the selection of analysis methods and the validity of results and supports the quality of the study in terms of research design and analysis. A clinical research coordinator who is in charge of the field research that forms the basis of the research, such as research registration and follow-up, is an important element in controlling the accuracy of cohort studies [28].
The involvement and training of researchers with expertise in clinical epidemiology research is essential for the smooth functioning of such a research organization and for ensuring the sustainability and development of cohort studies. In other words, researchers involved in cohort studies not only conduct statistical analysis using the constructed database and write manuscripts, they also should actively participate in the process of data collection and management and experience the entire process of clinical epidemiology research. Furthermore, they should participate in a series of clinical epidemiology research processes, from conceiving the research questions to specific research planning and protocol development. Through repetition of such practical learning, students will be encouraged to acquire the research execution skills to promote clinical research. Researchers who are skilled in clinical epidemiology research, and who have successfully completed a series of on-the-job training programs, form the most important basis for long-term follow-up of cohorts and addressing new developments.
Conclusion
In this review, we aimed to elucidate prognostic factors and validate the effects of treatment through the description and analysis of cohort studies conducted among patients with CKD. To extract high-quality evidence from observational databases that can lead to disease prevention and intervention studies, it is necessary to apply appropriate statistical analysis methods. The search for novel disease risk markers is now underway, and risk models incorporating risk markers that stratify specific populations must be created to verify the effectiveness of personalized treatment based on accurate individual risk prediction. For the long-term maintenance and development of cohort studies, in addition to the establishment of research organizations to ensure the quality of research, training of clinical researchers who are familiar with the process of clinical epidemiological research and who have the ability to manage practical affairs is required.
References
Lachat C, Hawwash D, Ocke MC, Berg C, Forsum E, Hornell A, Larsson C, Sonestedt E, Wirfalt E, Akesson A, Kolsteren P, Byrnes G, De Keyzer W, Van Camp J, Cade JE, Slimani N, Cevallos M, Egger M, Huybrechts I. Strengthening the reporting of observational studies in epidemiology-nutritional epidemiology (STROBE-nut): an extension of the STROBE statement. PLoS Med. 2016;13(6): e1002036. https://doi.org/10.1371/journal.pmed.1002036.
Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, Initiative S. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163–94. https://doi.org/10.7326/0003-4819-147-8-200710160-00010-w1.
Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, Initiative S. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–24. https://doi.org/10.1016/j.ijsu.2014.07.014.
Lea J, Greene T, Hebert L, Lipkowitz M, Massry S, Middleton J, Rostand SG, Miller E, Smith W, Bakris GL. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med. 2005;165(8):947–53. https://doi.org/10.1001/archinte.165.8.947.
Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–31.
Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–5. https://doi.org/10.1126/science.1193032.
Appel LJ, Wright JT Jr, Greene T, Kusek JW, Lewis JB, Wang X, Lipkowitz MS, Norris KC, Bakris GL, Rahman M, Contreras G, Rostand SG, Kopple JD, Gabbai FB, Schulman GI, Gassman JJ, Charleston J, Agodoa LY, African American Study of Kidney Disease and Hypertension Study Group. Long-term effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med. 2008;168(8):832–9. https://doi.org/10.1001/archinte.168.8.832.
Hu JR, Coresh J, Inker LA, Levey AS, Zheng Z, Rebholz CM, Tin A, Appel LJ, Chen J, Sarnak MJ, Grams ME. Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney Int. 2018;94(2):381–9. https://doi.org/10.1016/j.kint.2018.03.008.
Luo S, Coresh J, Tin A, Rebholz CM, Appel LJ, Chen J, Vasan RS, Anderson AH, Feldman HI, Kimmel PL, Waikar SS, Kottgen A, Evans AM, Levey AS, Inker LA, Sarnak MJ, Grams ME, Chronic Kidney Disease Biomarkers Consortium, Investigators. Serum metabolomic alterations associated with proteinuria in CKD. Clin J Am Soc Nephrol. 2019;14(3):342–53. https://doi.org/10.2215/CJN.10010818.
McCullough PA, Li S, Jurkovitz CT, Stevens LA, Wang C, Collins AJ, Chen SC, Norris KC, McFarlane SI, Johnson B, Shlipak MG, Obialo CI, Brown WW, Vassalotti JA, Whaley-Connell AT, Kidney Early Evaluation Program Investigators. CKD and cardiovascular disease in screened high-risk volunteer and general populations: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008;51(4 Suppl 2):S38-45. https://doi.org/10.1053/j.ajkd.2007.12.017.
Myers OB, Pankratz VS, Norris KC, Vassalotti JA, Unruh ML, Argyropoulos C. Surveillance of CKD epidemiology in the US - a joint analysis of NHANES and KEEP. Sci Rep. 2018;8(1):15900. https://doi.org/10.1038/s41598-018-34233-w.
Jolly SE, Burrows NR, Chen SC, Li S, Jurkovitz CT, Norris KC, Shlipak MG. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP). Clin J Am Soc Nephrol. 2011;6(8):1858–65. https://doi.org/10.2215/CJN.00500111.
Babayev R, Whaley-Connell A, Kshirsagar A, Klemmer P, Navaneethan S, Chen SC, Li S, McCullough PA, Bakris G, Bomback A, Keep Investigators. Association of race and body mass index with ESRD and mortality in CKD stages 3–4: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2013;61(3):404–12. https://doi.org/10.1053/j.ajkd.2012.11.038.
Jurkovitz CT, Li S, Norris KC, Saab G, Bomback AS, Whaley-Connell AT, McCullough PA, Keep Investigators. Association between lack of health insurance and risk of death and ESRD: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2013;61(4 Suppl 2):S24-32. https://doi.org/10.1053/j.ajkd.2012.12.015.
Hannan M, Ansari S, Meza N, Anderson AH, Srivastava A, Waikar S, Charleston J, Weir MR, Taliercio J, Horwitz E, Saunders MR, Wolfrum K, Feldman HI, Lash JP, Ricardo AC, CRIC Study Investigators; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Risk factors for CKD progression: overview of findings from the CRIC study. Clin J Am Soc Nephrol. 2021;16(4):648–59. https://doi.org/10.2215/CJN.07830520.
Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–15. https://doi.org/10.2215/CJN.01941205.
Eckardt KU, Barthlein B, Baid-Agrawal S, Beck A, Busch M, Eitner F, Ekici AB, Floege J, Gefeller O, Haller H, Hilge R, Hilgers KF, Kielstein JT, Krane V, Kottgen A, Kronenberg F, Oefner P, Prokosch HU, Reis A, Schmid M, Schaeffner E, Schultheiss UT, Seuchter SA, Sitter T, Sommerer C, Walz G, Wanner C, Wolf G, Zeier M, Titze S. The German Chronic Kidney Disease (GCKD) study: design and methods. Nephrol Dial Transplant. 2012;27(4):1454–60. https://doi.org/10.1093/ndt/gfr456.
Schneider MP, Hilgers KF, Schmid M, Hubner S, Nadal J, Seitz D, Busch M, Haller H, Kottgen A, Kronenberg F, Baid-Agrawal S, Schlieper G, Schultheiss U, Sitter T, Sommerer C, Titze S, Meiselbach H, Wanner C, Eckardt KU, GCKD Study Investigators. Blood pressure control in chronic kidney disease: a cross-sectional analysis from the German Chronic Kidney Disease (GCKD) study. PLoS ONE. 2018;13(8):e0202604. https://doi.org/10.1371/journal.pone.0202604.
Beck H, Titze SI, Hubner S, Busch M, Schlieper G, Schultheiss UT, Wanner C, Kronenberg F, Krane V, Eckardt KU, Kottgen A, GCKD Investigators. Heart failure in a cohort of patients with chronic kidney disease: the GCKD study. PLoS ONE. 2015;10(4):e0122552. https://doi.org/10.1371/journal.pone.0122552.
Heindel J, Baid-Agrawal S, Rebholz CM, Nadal J, Schmid M, Schaeffner E, Schneider MP, Meiselbach H, Kaesler N, Bergmann M, Ernst S, Krane V, Eckardt KU, Floege J, Schlieper G, Saritas T, GCKD Investigators. Association between dietary patterns and kidney function in patients with chronic kidney disease: a cross-sectional analysis of the German chronic kidney disease study. J Ren Nutr. 2020;30(4):296–304. https://doi.org/10.1053/j.jrn.2019.09.008.
Jing J, Kielstein JT, Schultheiss UT, Sitter T, Titze SI, Schaeffner ES, McAdams-DeMarco M, Kronenberg F, Eckardt KU, Kottgen A, GCKD Investigators. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant. 2015;30(4):613–21. https://doi.org/10.1093/ndt/gfu352.
Sekula P, Tin A, Schultheiss UT, Baid-Agrawal S, Mohney RP, Steinbrenner I, Yu B, Luo S, Boerwinkle E, Eckardt KU, Coresh J, Grams ME, Kttgen A. Urine 6-bromotryptophan: associations with genetic variants and incident end-stage kidney disease. Sci Rep. 2020;10(1):10018. https://doi.org/10.1038/s41598-020-66334-w.
Steubl D, Schneider MP, Meiselbach H, Nadal J, Schmid MC, Saritas T, Krane V, Sommerer C, Baid-Agrawal S, Voelkl J, Kotsis F, Kottgen A, Eckardt KU, Scherberich JE, GCKD Investigators. Association of serum uromodulin with death, cardiovascular events, and kidney failure in CKD. Clin J Am Soc Nephrol. 2020;15(5):616–24. https://doi.org/10.2215/CJN.11780919.
Jing J, Ekici AB, Sitter T, Eckardt KU, Schaeffner E, Li Y, Kronenberg F, Kottgen A, Schultheiss UT. Genetics of serum urate concentrations and gout in a high-risk population, patients with chronic kidney disease. Sci Rep. 2018;8(1):13184. https://doi.org/10.1038/s41598-018-31282-z.
Raschenberger J, Kollerits B, Titze S, Kottgen A, Barthlein B, Ekici AB, Forer L, Schonherr S, Weissensteiner H, Haun M, Wanner C, Eckardt KU, Kronenberg F, GCKD Investigators. Association of relative telomere length with cardiovascular disease in a large chronic kidney disease cohort: the GCKD study. Atherosclerosis. 2015;242(2):529–34. https://doi.org/10.1016/j.atherosclerosis.2015.08.020.
Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A. Chronic Kidney Disease Japan Cohort (CKD-JAC) study: design and methods. Hypertens Res. 2008;31(6):1101–7. https://doi.org/10.1291/hypres.31.1101.
Yamamoto T, Nakayama M, Miyazaki M, Matsushima M, Sato T, Taguma Y, Sato H, Ito S. Relationship between low blood pressure and renal/cardiovascular outcomes in Japanese patients with chronic kidney disease under nephrologist care: the Gonryo study. Clin Exp Nephrol. 2015. https://doi.org/10.1007/s10157-015-1084-4.
Tanaka S, Ninomiya T, Fujisaki K, Yoshida H, Nagata M, Masutani K, Tokumoto M, Mitsuiki K, Hirakata H, Fujimi S, Kiyohara Y, Kitazono T, Tsuruya K, Fukuoka Kidney disease Registry Study Collaboration Group. The Fukuoka Kidney disease Registry (FKR) Study: design and methods. Clin Exp Nephrol. 2017;21(3):465–73. https://doi.org/10.1007/s10157-016-1294-4.
Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol. 2003;14(12):3270–7. https://doi.org/10.1097/01.asn.0000100127.54107.57.
Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis. 2009;53(3):475–91. https://doi.org/10.1053/j.ajkd.2008.10.043.
Tentori F, Zhang J, Li Y, Karaboyas A, Kerr P, Saran R, Bommer J, Port F, Akiba T, Pisoni R, Robinson B. Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2012;27(11):4180–8. https://doi.org/10.1093/ndt/gfs021.
Saran R, Bragg-Gresham JL, Rayner HC, Goodkin DA, Keen ML, Van Dijk PC, Kurokawa K, Piera L, Saito A, Fukuhara S, Young EW, Held PJ, Port FK. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64(1):254–62. https://doi.org/10.1046/j.1523-1755.2003.00064.x.
Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85(1):174–81. https://doi.org/10.1038/ki.2013.327.
Fujisaki K, Joki N, Tanaka S, Kanda E, Hamano T, Masakane I, Tsuruya K. Pre-dialysis hyponatremia and change in serum sodium concentration during a dialysis session are significant predictors of mortality in patients undergoing hemodialysis. Kidney Int Rep. 2021;6(2):342–50.
Eriguchi R, Taniguchi M, Ninomiya T, Hirakata H, Fujimi S, Tsuruya K, Kitazono T. Hyporesponsiveness to erythropoiesis-stimulating agent as a prognostic factor in Japanese hemodialysis patients: the Q-Cohort study. J Nephrol. 2015;28(2):217–25. https://doi.org/10.1007/s40620-014-0121-9.
Tanaka S, Ninomiya T, Taniguchi M, Fujisaki K, Tokumoto M, Hirakata H, Ooboshi H, Kitazono T, Tsuruya K. Comparison of oral versus intravenous vitamin D receptor activator in reducing infection-related mortality in hemodialysis patients: the Q-Cohort Study. Nephrol Dial Transplant. 2016;31(7):1152–60. https://doi.org/10.1093/ndt/gfw205.
Yamada S, Tsuruya K, Taniguchi M, Tokumoto M, Fujisaki K, Hirakata H, Fujimi S, Kitazono T. Association between serum phosphate levels and stroke risk in patients undergoing hemodialysis: the Q-Cohort Study. Stroke. 2016;47(9):2189–96. https://doi.org/10.1161/STROKEAHA.116.013195.
Yotsueda R, Taniguchi M, Tanaka S, Eriguchi M, Fujisaki K, Torisu K, Masutani K, Hirakata H, Kitazono T, Tsuruya K. Cardiothoracic ratio and all-cause mortality and cardiovascular disease events in hemodialysis patients: the Q-Cohort Study. Am J Kidney Dis. 2017;70(1):84–92. https://doi.org/10.1053/j.ajkd.2016.11.026.
Yamada S, Taniguchi M, Tokumoto M, Yoshitomi R, Yoshida H, Tatsumoto N, Hirakata H, Fujimi S, Kitazono T, Tsuruya K. Modified creatinine index and the risk of bone fracture in patients undergoing hemodialysis: the Q-Cohort Study. Am J Kidney Dis. 2017;70(2):270–80. https://doi.org/10.1053/j.ajkd.2017.01.052.
Tanaka S, Ninomiya T, Hiyamuta H, Taniguchi M, Tokumoto M, Masutani K, Ooboshi H, Nakano T, Tsuruya K, Kitazono T. Apparent treatment-resistant hypertension and cardiovascular risk in hemodialysis patients: ten-year outcomes of the Q-Cohort Study. Sci Rep. 2019;9(1):1043. https://doi.org/10.1038/s41598-018-37961-1.
Tanaka S, Nakano T, Hiyamuta H, Taniguchi M, Tokumoto M, Masutani K, Ooboshi H, Tsuruya K, Kitazono T. Impact of multivascular disease on cardiovascular mortality and morbidity in patients receiving hemodialysis: ten-year outcomes of the Q-Cohort Study. J Atheroscler Thromb. 2020. https://doi.org/10.5551/jat.54098.
Tanaka S, Ninomiya T, Katafuchi R, Masutani K, Tsuchimoto A, Noguchi H, Hirakata H, Tsuruya K, Kitazono T. Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin J Am Soc Nephrol. 2013;8(12):2082–90. https://doi.org/10.2215/CJN.03480413.
Ueki K, Tsuchimoto A, Matsukuma Y, Nakagawa K, Tsujikawa H, Masutani K, Tanaka S, Kaku K, Noguchi H, Okabe Y, Unagami K, Kakuta Y, Okumi M, Nakamura M, Tsuruya K, Nakano T, Tanabe K, Kitazono T, Japan Academic Consortium of Kidney Transplantation i. Development and validation of a risk score for the prediction of cardiovascular disease in living donor kidney transplant recipients. Nephrol Dial Transplant. 2021;36(2):365–74. https://doi.org/10.1093/ndt/gfaa275.
Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O’Donnell CJ, Smith SC Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119(17):2408–16. https://doi.org/10.1161/CIRCULATIONAHA.109.192278.
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. https://doi.org/10.1002/sim.2929 (discussion 207-12).
Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. https://doi.org/10.1002/sim.4085.
Tanaka S, Ninomiya T, Masutani K, Nagata M, Tsuchimoto A, Tsuruya K, Kitazono T. Prognostic impact of serum bilirubin level on long-term renal survival in IgA nephropathy. Clin Exp Nephrol. 2015;19(6):1062–70. https://doi.org/10.1007/s10157-015-1096-0.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Rassen JA, Schneeweiss S, Glynn RJ, Mittleman MA, Brookhart MA. Instrumental variable analysis for estimation of treatment effects with dichotomous outcomes. Am J Epidemiol. 2009;169(3):273–84. https://doi.org/10.1093/aje/kwn299.
Acknowledgements
The authors thank the participants in the FKR study, the members of the FKR Study Group, and all personnel at participating institutions involved in the study. We thank Satoru Fujimi (Fukuoka Renal Clinic), Hideki Hirakata (Fukuoka Renal Clinic), Tadashi Hirano (Hakujyuji Hospital), Tetsuhiko Yoshida (Hamanomachi Hospital), Takashi Deguchi (Hamanomachi Hospital), Hideki Yotsueda (Harasanshin Hospital), Kiichiro Fujisaki (Iizuka Hospital), Keita Takae (Japanese Red Cross Fukuoka Hospital), Koji Mitsuiki (Japanese Red Cross Fukuoka Hospital), Akinori Nagashima (Japanese Red Cross Karatsu Hospital), Ritsuko Katafuchi (Kano Hospital), Hidetoshi Kanai (Kokura Memorial Hospital), Kenji Harada (Kokura Memorial Hospital), Tohru Mizumasa (Kyushu Central Hospital), Takanari Kitazono (Kyushu University), Toshiaki Nakano (Kyushu University), Toshiharu Ninomiya (Kyushu University), Kumiko Torisu (Kyushu University), Akihiro Tsuchimoto (Kyushu University), Shunsuke Yamada (Kyushu University), Hiroto Hiyamuta (Kyushu University), Shigeru Tanaka (Kyushu University), Dai Matsuo (Munakata Medical Association Hospital), Yusuke Kuroki (National Fukuoka-Higashi Medical Center), Hiroshi Nagae (National Fukuoka-Higashi Medical Center), Masaru Nakayama (National Kyushu Medical Center), Kazuhiko Tsuruya (Nara Medical University), Masaharu Nagata (Shin-eikai Hospital), Taihei Yanagida (Steel Memorial Yawata Hospital), Shotaro Onaka (Tagawa Municipal Hospital). We thank Analisa Avila, ELS, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript. I would like to sincerely thank the Japanese Society of Nephrology for this Clinical Scientist Award. The authors would like to express their appreciation to the following investigators at the participating institutions: we thank the participants in the Q-Cohort Study, members of the Society for the Study of Kidney Disease, and all personnel at participating institutions involved in the study. The following personnel (institutions) participated in the study: Takashi Ando (Hakozaki Park Internal Medicine Clinic), Takashi Ariyoshi (Ariyoshi Clinic), Koichiro Goto (Goto Clinic), Fumitada Hattori (Nagao Hospital), Harumichi Higashi (St. Mary’s Hospital), Tadashi Hirano (Hakujyuji Hospital), Kei Hori (Munakata Medical Association Hospital), Takashi Inenaga (Ekisaikai Moji Hospital), Hidetoshi Kanai (Kokura Memorial Hospital), Shigemi Kiyama (Kiyama Naika), Tetsuo Komota (Komota Clinic), Hiromasa Kuma (Kuma Clinic), Toshiro Maeda (Kozenkai–Maeda Hospital), Junichi Makino (Makino Clinic), Dai Matsuo (Hirao Clinic), Chiaki Miishima (Miishima Naika Clinic), Koji Mitsuiki (Japanese Red Cross Fukuoka Hospital), Kenichi Motomura (Motomura Naika Clinic), Sadatoshi Nakamura and Hidetoshi Nakamura (Kokura Daiichi Hospital), Koichi Nakashima (Ohashi Internal Circulatory Clinic), Nobumitsu Okita (Shiroishi Kyoritsu Hospital), Shinichiro Osato (Osato Jin Clinic), Sakura Sakamoto (Fujiyamato Spa Hospital), Keiko Shigematsu (Shigematsu Clinic), Kazumasa Shimamatsu (Shimamatsu Naika Iin), Yoshito Shogakiuchi (Shin-Ai Clinic), Hiroaki Takamura (Hara Hospital), Kazuhito Takeda (Iizuka Hospital), Asuka Terai (Chidoribashi Hospital), Hideyoshi Tanaka (Mojiko-Jin Clinic), Suguru Tomooka (Hakozaki Park Internal Medicine Clinic), Jiro Toyonaga (Fukuoka Renal Clinic), Hiroshi Tsuruta (Steel Memorial Yawata Hospital), Ryutaro Yamaguchi (Shiseikai Hospital), Taihei Yanagida (Saiseikai Yahata General Hospital), Tetsuro Yanase (Yanase Internal Medicine Clinic), Tetsuhiko Yoshida (Hamanomachi Hospital), Takahiro Yoshimitsu (Gofukumachi Kidney Clinic, Harasanshin Hospital), and Koji Yoshitomi (Yoshitomi Medical Clinic).
Funding
This study was supported by the Kidney Foundation (H19 JKFB 07-13, H20 JKFB 08-8, H23 JKFB 11-11) and the Japan Dialysis Outcome Research Foundation (H19-076-02, H20-003). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
Shigeru Tanaka contributed to drafting of the manuscript. Toshiaki Nakano, Kazuhiko Tsuruya, and Takanari Kitazono contributed to critical revision of the manuscript and supervision. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was presented as the Clinical Scientist Award memorial lecture at the 63rd annual meeting of the Japanese Society of Nephrology, held at Yokohama, Japan in 2020.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tanaka, S., Nakano, T., Tsuruya, K. et al. Clinical epidemiological analysis of cohort studies investigating the pathogenesis of kidney disease. Clin Exp Nephrol 26, 1–12 (2022). https://doi.org/10.1007/s10157-021-02121-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-021-02121-9