Abstract
Background
Granulocyte colony-stimulating factor (G-CSF) reportedly reduces the risk of neutropenia and subsequent infections caused by cancer chemotherapy. Although several guidelines recommend using G-CSF in primary prophylaxis according to the incidence rate of chemotherapy-induced febrile neutropenia (FN), the effectiveness of G-CSF in digestive system tumor chemotherapy remains unclear. To address these clinical questions, we conducted a systematic review as part of revising the Clinical Practice Guidelines for the Use of G-CSF 2022 published by the Japan Society of Clinical Oncology.
Methods
This systematic review addressed two main clinical questions (CQ): CQ1: “Is primary prophylaxis with G-CSF effective in chemotherapy?”, and CQ2: “Is increasing the intensity of chemotherapy with G-CSF effective?” We reviewed different types of digestive system tumors, including esophageal, gastric, pancreatic, biliary tract, colorectal, and neuroendocrine carcinomas. PubMed, Cochrane Library, and Ichushi-Web databases were searched for information sources. Independent systematic reviewers conducted two rounds of screening and selected relevant records for each CQ. Finally, the working group members synthesized the strength of evidence and recommendations.
Results
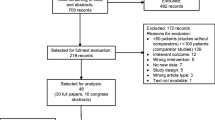
After two rounds of screening, 5/0/3/0/2/0 records were extracted for CQ1 of esophageal/gastric/pancreatic/biliary tract/colorectal/ and neuroendocrine carcinoma, respectively. Additionally, a total of 2/6/1 records were extracted for CQ2 of esophageal/pancreatic/colorectal cancer, respectively. The strength of evidence and recommendations were evaluated for CQ1 of colorectal cancer; however, we could not synthesize recommendations for other CQs owing to the lack of records.
Conclusion
The use of G-CSF for primary prophylaxis in chemotherapy for colorectal cancer is inappropriate.

Similar content being viewed by others
References
Cancer Information Service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed 29 Jan 2024
Del Mastro L, De Placido S, Bruzzi P et al (2015) Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet 385:1863–1872. https://doi.org/10.1016/S0140-6736(14)62048-1
The Japan Society of Clinical Oncology (2022) Clinical Practice Guidelines for the Use of G-CSF. KANEHARA & CO., LTD.
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol 33:3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32. https://doi.org/10.1016/j.ejca.2010.10.013
NCCN Clinical Practice Guideline: hematopoietic growth factors. Version 2, 2024. Accessed 30 Dec 30 2023
Andrews J, Guyatt G, Oxman AD et al (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 66:719–725. https://doi.org/10.1016/j.jclinepi.2012.03.013
Andrews JC, Schünemann HJ, Oxman AD et al (2013) GRADE guidelines: 15. Going from evidence to recommendation—determinants of a recommendation’s direction and strength. J Clin Epidemiol 66:726–735. https://doi.org/10.1016/j.jclinepi.2013.02.003
Wu YL, Yang JCH, Kim DW et al (2018) Phase II study of crizotinib in east asian patients with ROS1-positive advanced non–small-cell lung cancer. J Clin Oncol 36:1405–1411. https://doi.org/10.1200/JCO.2017.75.5587
Yoshida Y, Komori K, Aoki M et al (2018) Efficacy of pegfilgrastim administration in patients with esophageal cancer treated with docetaxel, cisplatin, and 5-fluorouracil. Pharmazie 73:613–616. https://doi.org/10.1691/ph.2018.8576
Kawahira M, Yokota T, Hamauchi S et al (2018) Primary prophylactic granulocyte colony-stimulating factor according to ASCO guidelines has no preventive effect on febrile neutropenia in patients treated with docetaxel, cisplatin, and 5-fluorouracil chemotherapy. Int J Clin Oncol 23:1189–1195. https://doi.org/10.1007/s10147-018-1306-3
Ohkura Y, Ueno M, Udagawa H (2019) Risk factors for febrile neutropenia and effectiveness of primary prophylaxis with pegfilgrastim in patients with esophageal cancer treated with docetaxel, cisplatin, and 5-fluorouracil. World J Surg Oncol 17:1–6. https://doi.org/10.1186/s12957-019-1665-x
Kang YK, Chen LT, Ryu MH et al (2022) Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-contr. Lancet Oncol 23:234–247. https://doi.org/10.1016/S1470-2045(21)00692-6
Janjigian YY, Shitara K, Moehler M et al (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398:27–40. https://doi.org/10.1016/S0140-6736(21)00797-2
Shitara K, Bang Y-J, Iwasa S et al (2020) Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 382:2419–2430. https://doi.org/10.1056/nejmoa2004413
Moorcraft SY, Khan K, Peckitt C et al (2014) FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: the Royal Marsden experience. Clin Colorectal Cancer 13:232–238. https://doi.org/10.1016/j.clcc.2014.09.005
Ninomiya R, Nakazawa A, Miyata Y et al (2016) Primary prophylactic administration of pegfilgrastim in FOLFIRINOX therapy for locally advanced pancreatic carcinoma. Gan To Kagaku Ryoho 43:1678–1680
Lee J, Kim JW, Ahn S et al (2017) Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: using cumulative relative dose intensity. Eur J Cancer 76:125–133. https://doi.org/10.1016/j.ejca.2017.02.010
Pinter T, Klippel Z, Cesas A et al (2017) A phase III, randomized, double-blind, placebo-controlled trial of pegfilgrastim in patients receiving first-line FOLFOX/bevacizumab or FOLFIRI/bevacizumab for locally advanced or metastatic colorectal cancer: final results of the pegfilgrastim and anti-V. Clin Colorectal Cancer 16:103-114.e3. https://doi.org/10.1016/j.clcc.2016.08.008
Hecht JR, Pillai M, Gollard R et al (2010) A randomized, placebo-controlled phase II study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin Colorectal Cancer 9:95–101. https://doi.org/10.3816/CCC.2010.n.013
Ishikawa T, Yasuda T, Okayama T et al (2019) Early administration of pegfilgrastim for esophageal cancer treated with docetaxel, cisplatin, and fluorouracil: a phase II study. Cancer Sci 110:3754–3760. https://doi.org/10.1111/cas.14218
Sun J-M, Shen L, Shah MA et al (2021) Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398:759–771. https://doi.org/10.1016/s0140-6736(21)01234-4
Kato K, Ito Y, Daiko H et al (2022) A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol 40:238. https://doi.org/10.1200/JCO.2022.40.4_suppl.238
Pleasance E, Bohm A, Williamson LM et al (2022) Whole-genome and transcriptome analysis enhances precision cancer treatment options. Ann Oncol 33:939–949. https://doi.org/10.1016/j.annonc.2022.05.522
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Okusaka T, Ikeda M, Fukutomi A et al (2014) Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci 105:1321–1326. https://doi.org/10.1111/cas.12501
Ozaka M, Ishii H, Sato T et al (2018) A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 81:1017–1023. https://doi.org/10.1007/s00280-018-3577-9
Morizane C, Okusaka T, Mizusawa J et al (2019) Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol 30:1950–1958. https://doi.org/10.1093/annonc/mdz402
Ioka T, Kanai M, Kobayashi S et al (2022) Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401-MITSUBA). J Hepatobiliary Pancreat Sci 30:102–110. https://doi.org/10.1002/jhbp.1219
Oki E, Kato T, Bando H et al (2018) A multicenter clinical phase II study of FOLFOXIRI plus bevacizumab as first-line therapy in patients with metastatic colorectal cancer: QUATTRO study. Clin Colorectal Cancer 17:147–155. https://doi.org/10.1016/j.clcc.2018.01.011
Shinozaki K, Yamada T, Nasu J et al (2021) A phase II study of FOLFOXIRI plus bevacizumab as initial chemotherapy for patients with untreated metastatic colorectal cancer: TRICC1414 (BeTRI). Int J Clin Oncol 26:399–408. https://doi.org/10.1007/s10147-020-01811-w
Cremolini C, Loupakis F, Antoniotti C et al (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16:1306–1315. https://doi.org/10.1016/S1470-2045(15)00122-9
Satake H, Sunakawa Y, Miyamoto Y et al (2018) A phase II trial of 1st-line modified-FOLFOXIRI plus bevacizumab treatment for metastatic colorectal cancer harboring RAS mutation: JACCRO CC-11. Oncotarget 9:18811–18820. https://doi.org/10.18632/oncotarget.24702
Morizane C, Machida N, Honma Y et al (2022) Effectiveness of etoposide and cisplatin vs irinotecan and cisplatin therapy for patients with advanced neuroendocrine carcinoma of the digestive system. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2022.3395
Acknowledgements
This guideline and systematic review were led by the Japanese Society of Clinical Oncology (JSCO). The authors thank Mr. Naohiko Yamaguchi and Ms. Yuko Mitsuoka for the initial record search and Professor Masahiro Yoshida of the Minds Tokyo GRADE Center for imparting knowledge about the systematic review methodology. We would also like to thank Ms. Natsuki Fukuda for her efforts in managing the project and formulating the guidelines.
Author information
Authors and Affiliations
Contributions
Conceptualization: T. T. and K. T.; Formal analysis: M. I., Y. O., K. N., E. B., and K. T.; Investigation: M. I., Y. O., K. N., E. B., and K. T.; Data curation: M. I., Y. O., K. N., E. B., and K. T.; Writing—original draft preparation: M. I.; Writing—review and editing: Y. O., K. N., E. B., and K. T.; Visualization: M. I.; Supervision: all authors. Project administration: T. T. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Eishi Baba received honoraria from Chugai Pharmaceutical Co., Ltd. and Daiichi Sankyo Co., Ltd., and research funding from Taiho Pharmaceutical Co., Ltd. and Chugai Pharmaceutical Co., Ltd. Yukinori Ozaki received honoraria from Daiichi Sankyo Co., Ltd., Pfizer Japan Inc., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., and Kyowa Kirin Co., Ltd. Eiki Ichihara received honoraria from Eli Lilly Japan K.K. and research funding from MSD K.K., Ono Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., and Takeda Pharmaceutical Co., Ltd. Yuji Miura received honoraria from Ono Pharmaceutical Co., Ltd., MSD K.K., Takeda Pharmaceutical Co., Ltd., Eisai Co., Ltd., and Bristol Myers Squibb Co., Ltd., and research funding from MSD K.K. and Ono Pharmaceutical Co., Ltd. Shingo Yano received research funding from Otsuka Pharmaceutical Co., Ltd. Dai Maruyama received honoraria from Ono Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Nippon Shinyaku Co., Ltd., Eisai Co., Ltd., Mundipharma, Kyowa Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Zenyaku Holdings Co., Ltd., MSD K.K., SymBio Pharmaceutical Ltd., Sanofi K.K., AbbVie Inc., Takeda Pharmaceutical Co., Ltd., AstraZeneca K.K., Bristol Myers Squibb Co., Ltd., and Genmab K.K., and research funding from Amgen K.K., Astellas Pharma Inc., Biopharma Co., Ltd., Novartis Pharmaceutical K.K., Kyowa Kirin Co., Ltd., Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., Bristol Myers Squibb Co., Ltd., AbbVie Inc., Eisai Co., Ltd., MSD K.K., Taiho Pharmaceutical Co., Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., and Genmab K.K. Tetsuhiro Yoshinami received honoraria from Kyowa Kirin Co., Ltd., Pfizer Japan Inc., Chugai Pharmaceutical Co., Eli Lilly Japan K.K., MSD K.K., AstraZeneca K.K., and Eisai Co., Ltd. Takashi Motohashi received honoraria from AstraZeneca K. K., Chugai Pharmaceutical Co., and Myriad Genetics G. K. Toshio Kubo has received honoraria from Chugai Pharmaceutical Co. Takahiro Kimura received honoraria from Sanofi K.K. Shinji Nakao received honoraria from Kyowa Kirin Co., Ltd. Atsushi Sato received honoraria from Chugai Pharmaceutical Co., and Taiho Pharmaceutical Co., Ltd., and received scholarship donations from Chugai Pharmaceutical Co., and Taiho Pharmaceutical Co., Ltd. Toshimi Takano received honoraria from Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., and Eli Lilly Japan K.K. Kenji Tsuchihashi received honoraria from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Taiho Pharmaceutical Co., Ltd., and Novartis Pharmaceutical K.K.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ito, M., Okumura, Y., Nio, K. et al. Effectiveness of G-CSF in chemotherapy for digestive system tumors: a systematic review of the Clinical Practice Guidelines for the Use of G-CSF 2022 delineated by the Japan Society of Clinical Oncology. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02502-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02502-6