Abstract
Purpose
We assessed clinical outcomes in patients with metastatic castration-sensitive prostate cancer (mCSPC) treated with two upfront therapies.
Methods
The medical records of 301 patients with mCSPC treated with androgen deprivation therapy plus upfront abiraterone acetate (ABI) or docetaxel (DOC) between 2014 and 2021 were retrospectively reviewed. Propensity score matching (PSM) was performed to compare survival outcomes. Subgroup analyses of risk factors for second progression were conducted.
Results
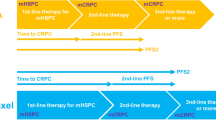
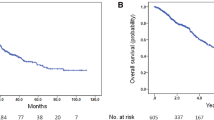
A total of 95 patients received upfront DOC, whereas 206 received upfront ABI. After PSM, the ABI group had a significantly better castration-resistant prostate cancer (CRPC)-free survival than the DOC group [hazard ratio (HR), 0.53; 95% confidence interval (CI), 0.34–0.82]. Second progression-free survival (PFS2) tended to be longer in the ABI group than in the DOC group, but the difference was not statistically significant (HR, 0.64; 95% CI, 0.33–1.22). No significant difference in overall survival (OS) was found between the two groups (HR, 0.92; 95% CI, 0.42–2.03). In the subgroup analysis, upfront ABI had significantly favorable PFS2 in patients aged ≥ 75 years compared with upfront DOC (p = 0.038). Four risk factors for second progression (primary Gleason 5, liver metastasis, high serum alkaline phosphatase level, and high serum lactate dehydrogenase level) successfully stratified patients into three risk groups.
Conclusions
Upfront ABI provided better CRPC-free survival than upfront DOC; however, no significant differences in PFS2 or OS were observed between the two groups. Personalized management based on prognostic risk factors may benefit patients with mCSPC treated with upfront intensified therapies.



Similar content being viewed by others
References
Ferlay J, Colombet M, Soerjomataram I et al (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144(8):1941–1953. https://doi.org/10.1002/ijc.31937
Mosillo C, Iacovelli R, Ciccarese C et al (2018) De novo metastatic castration sensitive prostate cancer: State of art and future perspectives. Cancer Treat Rev 70:67–74. https://doi.org/10.1016/j.ctrv.2018.08.005
Gaylis FD, Choi JE, Hamilton Z et al (2017) Change in prostate cancer presentation coinciding with USPSTF screening recommendations at a community-based urology practice. Urol Oncol 35(11):663.e661-663.e667. https://doi.org/10.1016/j.urolonc.2017.06.059
Chen R, Ren S, Yiu MK et al (2014) Prostate cancer in Asia: A collaborative report. Asian J Urol 1(1):15–29. https://doi.org/10.1016/j.ajur.2014.08.007
Sweeney CJ, Chen YH, Carducci M et al (2015) Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 373(8):737–746. https://doi.org/10.1056/NEJMoa1503747
Barata PC, Sartor AO (2019) Metastatic castration-sensitive prostate cancer: Abiraterone, docetaxel, or. Cancer 125(11):1777–1788. https://doi.org/10.1002/cncr.32039
Fizazi K, Tran N, Fein L et al (2017) Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 377(4):352–360. https://doi.org/10.1056/NEJMoa1704174
Parker CC, James ND, Brawley CD et al (2018) Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. https://doi.org/10.1016/s0140-6736(18)32486-3
Davis ID, Martin AJ, Stockler MR et al (2019) Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 381(2):121–131. https://doi.org/10.1056/NEJMoa1903835
Sydes MR, Spears MR, Mason MD et al (2018) Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol 29(5):1235–1248. https://doi.org/10.1093/annonc/mdy072
Marchioni M, Di Nicola M, Primiceri G et al (2020) New antiandrogen compounds compared to docetaxel for metastatic hormone sensitive prostate cancer: results from a network meta-analysis. J Urol 203(4):751–759. https://doi.org/10.1097/ju.0000000000000636
Sathianathen NJ, Koschel S, Thangasamy IA et al (2020) Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur Urol 77(3):365–372. https://doi.org/10.1016/j.eururo.2019.09.004
Mori K, Mostafaei H, Sari Motlagh R et al (2021) Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU Int. https://doi.org/10.1111/bju.15507
S N (2022) Real-world survival outcomes of adding docetaxel or abiraterone in patients with high-volume metastatic castration-sensitive prostate cancer: historically controlled, propensity score matched comparison with androgen deprivation therapy. World J Urol in press
Scher HI, Halabi S, Tannock I et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26(7):1148–1159
Glass TR, Tangen CM, Crawford ED et al (2003) Metastatic carcinoma of the prostate: identifying prognostic groups using recursive partitioning. J Urol 169(1):164–169. https://doi.org/10.1097/01.ju.0000042482.18153.30
Miyoshi Y, Noguchi K, Yanagisawa M et al (2015) Nomogram for overall survival of Japanese patients with bone-metastatic prostate cancer. BMC Cancer 15:338. https://doi.org/10.1186/s12885-015-1330-x
Narita S, Hatakeyama S, Takahashi M et al (2020) Clinical outcomes and prognostic factors in patients with newly diagnosed metastatic prostate cancer initially treated with androgen deprivation therapy: a retrospective multicenter study in Japan. Int J Clin Oncol. https://doi.org/10.1007/s10147-019-01614-8
Akamatsu S, Kubota M, Uozumi R et al (2019) Development and Validation of a Novel Prognostic Model for Predicting Overall Survival in Treatment-naïve Castration-sensitive Metastatic Prostate Cancer. Eur Urol Oncol 2(3):320–328. https://doi.org/10.1016/j.euo.2018.10.011
Sugihara M (2010) Survival analysis using inverse probability of treatment weighted methods based on the generalized propensity score. Pharm Stat 9(1):21–34. https://doi.org/10.1002/pst.365
King G, Nielsen R (2019) Why Propensity Scores Should Not Be Used for Matching. Polit Anal 27(4):435–454. https://doi.org/10.1017/pan.2019.11
Chowdhury S, Mainwaring P, Zhang L et al (2020) Systematic Review and Meta-Analysis of Correlation of Progression-Free Survival-2 and Overall Survival in Solid Tumors. Front Oncol. https://doi.org/10.3389/fonc.2020.01349
Lorente D, Castro E, Lozano R et al (2020) Association Between Second Progression-free Survival (PFS2) and Overall Survival in Metastatic Castration-resistant Prostate Cancer. Eur Urol 77(6):763–766. https://doi.org/10.1016/j.eururo.2020.02.025
Briones J, Khan M, Sidhu AK et al (2021) Population-Based Study of Docetaxel or Abiraterone Effectiveness and Predictive Markers of Progression Free Survival in Metastatic Castration-Sensitive Prostate Cancer. Front Oncol 11:658331. https://doi.org/10.3389/fonc.2021.658331
Smith MR, Rathkopf DE, Mulders PF et al (2015) Efficacy and Safety of Abiraterone Acetate in Elderly (75 Years or Older) Chemotherapy Naïve Patients with Metastatic Castration Resistant Prostate Cancer. J Urol 194(5):1277–1284. https://doi.org/10.1016/j.juro.2015.07.004
Mulders PF, Molina A, Marberger M et al (2014) Efficacy and safety of abiraterone acetate in an elderly patient subgroup (aged 75 and older) with metastatic castration-resistant prostate cancer after docetaxel-based chemotherapy. Eur Urol 65(5):875–883. https://doi.org/10.1016/j.eururo.2013.09.005
Horgan AM, Seruga B, Pond GR et al (2014) Tolerability and efficacy of docetaxel in older men with metastatic castrate-resistant prostate cancer (mCRPC) in the TAX 327 trial. J Geriatr Oncol 5(2):119–126. https://doi.org/10.1016/j.jgo.2013.12.001
Boyle HJ, Alibhai S, Decoster L et al (2019) Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer 116:116–136. https://doi.org/10.1016/j.ejca.2019.04.031
Takahara K, Naiki T, Ito T et al (2021) Useful predictors of progression-free survival for Japanese patients with LATITUDE-high-risk metastatic castration-sensitive prostate cancer who received upfront abiraterone acetate. Int J Urol. https://doi.org/10.1111/iju.14754
Pond GR, Sonpavde G, de Wit R et al (2014) The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol 65(1):3–6. https://doi.org/10.1016/j.eururo.2013.09.024
Isaksson J, Green H, Papantoniou D et al (2021) Real-world evaluation of upfront docetaxel in metastatic castration-sensitive prostate cancer. World J Clin Oncol 12(11):1009–1022. https://doi.org/10.5306/wjco.v12.i11.1009
Okamoto T, Noro D, Hatakeyama S et al (2021) Impact of pretreatment anemia on upfront abiraterone acetate therapy for metastatic hormone-sensitive prostate cancer: a multicenter retrospective study. BMC Cancer 21(1):605. https://doi.org/10.1186/s12885-021-08206-8
Sayegh N, Swami U, Agarwal N (2021) Recent Advances in the Management of Metastatic Prostate Cancer. JCO Oncol Pract. https://doi.org/10.1200/op.21.00206
Acknowledgements
We express our appreciation to Yukiko Sugiyama, Ken Watanabe, Nana Tomatsu, and Saeko Nakamura for their assistance in performing this study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-profit sectors.
Author information
Authors and Affiliations
Contributions
Shintaro Narita: data collection, study design, statistical analysis, and manuscript writing. Takahiro Kimura and Shingo Hatakeyama: study design and data collection. Kenichi Hata, Takafumi Yanagisawa, Shinya Maita, Shuji Chiba, Hiromi Sato, Soki Kashima, Atsushi Koizumi, Ryohei Yamamoto, Koichiro Takayama, Katsumi Okane, Toshiya Ishida, Yohei Horikawa, Teruaki Kumazawa, Jiro Shimoda, and Takehiro Suzuki: data collection. Kyoko Nomura: statistical analysis. Chikara Ohyama and Shin Egawa: supervision. Tomonori Habuchi: supervision and manuscript writing. All authors had read and approve of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Shintaro Narita received honoraria from Janssen Pharmaceutical K.K. Takahiro Kimura is a paid consultant/advisor of Astellas Pharma Inc., Bayer AG, Janssen Pharmaceutical K.K and Sanofi S.A. Shin Egawa is a paid consultant/advisor of Takeda, Astellas, AstraZeneca, Sanofi, Janssen, and Pfizer. Shingo Hatakeyama received honoraria from Janssen Pharmaceutical K.K. and Pfizer Inc. and Nipro Corporation. Chikara Ohyama received honoraria from Astellas Pharma Inc., NIPPON SHINYAKU Company Ltd., AstraZeneca K.K., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Company Ltd., Novartis Pharma K.K., ONO Pharmaceutical Company Ltd., Chugai Pharmaceutical Company Ltd., Sanofi S.A., Bayer AG., Pfizer Inc., Bristol Myers Squibb, Otsuka Pharmaceutical Company Ltd., KISSEI Pharmaceutical Company Ltd., Kyowa Kirin Company Ltd., Daiichi Sankyo Company Ltd., KANEKA Corporation, and Nipro Corporation. Tomonori Habuchi also received honoraria from Janssen Pharmaceutical K.K., Takeda Pharmaceutical Company Ltd., Astellas Pharma Inc., Daiichi Sankyo Company, Ltd., AstraZeneca K.K., Sanofi S.A., and Bayer AG. The other authors have no disclosures.
Informed consent
All patients gave opt-out consent for inclusion after being informed of the study and provided information on the institution’s website.
Research involving human participants and/or animals
The study involves human participants.
Ethical approval
The retrospective multicenter study was approved by each institutional review board and representative facility (A2018-03).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Narita, S., Kimura, T., Hatakeyama, S. et al. Real-world outcomes and risk stratification in patients with metastatic castration-sensitive prostate cancer treated with upfront abiraterone acetate and docetaxel. Int J Clin Oncol 27, 1477–1486 (2022). https://doi.org/10.1007/s10147-022-02203-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02203-y