Abstract
Background
The association between sarcopenia and prognosis in patients with locally advanced non-small cell lung cancer (NSCLC) undergoing trimodality therapy, consisting of preoperative concurrent chemoradiotherapy and surgery, has not been reported. Therefore, we aimed to investigate the association of sarcopenia and fat mass with prognosis after trimodality therapy.
Methods
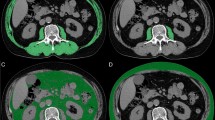
To assess sarcopenia, the psoas muscle mass was measured. Using computed tomography data, including third lumbar vertebra level images, psoas muscle mass and visceral and subcutaneous fat mass were measured. Additionally, body mass indices, and visceral/subcutaneous fat ratio, obtained by dividing the visceral fat index by the subcutaneous fat index, were calculated. We investigated the relationship between these parameters and overall survival.
Results
Ninety-nine eligible patients were included. In the univariate analysis, age, clinical stage, tumor location, psoas muscle index, and visceral/subcutaneous fat ratio were significant prognostic factors for overall survival (P = 0.008, P = 0.04, P = 0.04, P = 0.02, and P = 0.02, respectively). In the multivariate analysis, age and psoas muscle index were significant prognostic factors for overall survival (P = 0.01 and P = 0.03, respectively). The 5-year overall survival rates for the high and low psoas muscle index groups were 79.6% [95% confidence interval (CI), 67.1–94.5%] and 66.2% (95% CI, 54.1–81.1%), respectively; whereas, the 10-year overall survival rates were 61.9% (95% CI, 42.0–91.4%) and 25.3% (95% CI, 8.6–74.2%), respectively.
Conclusion
Sarcopenia was related to poor overall survival in patients with locally advanced NSCLC undergoing trimodality therapy. Assessment of body composition prior to treatment may provide important information for formulating rational therapeutic strategies.




Similar content being viewed by others
Data availability
The institution’s review board prohibits data sharing.
References
Ferlay J, Shin HR, Bray F et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Allemani C, Matsuda T, Di Carlo V et al (2018) Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391:1023–1075
Albain KS, Swann RS, Rusch VW et al (2009) Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 374:379–386
Toyooka S, Kiura K, Shien K et al (2012) Induction chemoradiotherapy is superior to induction chemotherapy for the survival of non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg 15:954–960
Shien K, Toyooka S, Soh J et al (2015) Lower lobe origin is a poor prognostic factor in locally advanced non-small-cell lung cancer patients treated with induction chemoradiotherapy. Mol Clin Oncol 3:706–712
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990s–991s
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Chen LK, Liu LK, Woo J et al (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101
Nishikawa H, Shiraki M, Hiramatsu A et al (2016) Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 46:951–963
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Yang M, Shen Y, Tan L et al (2019) Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest 156:101–111
Buentzel J, Heinz J, Bleckmann A et al (2019) Sarcopenia as prognostic factor in lung cancer patients: a systematic review and meta-analysis. Anticancer Res 39:4603–4612
Nishimura JM, Ansari AZ, D’Souza DM et al (2019) Computed tomography-assessed skeletal muscle mass as a predictor of outcomes in lung cancer surgery. Ann Thorac Surg 108:1555–1564
Deng HY, Hou L, Zha P et al (2019) Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: a comprehensive systematic review and meta-analysis. Eur J Surg Oncol 45:728–735
Shinohara S, Otsuki R, Kobayashi K et al (2020) Impact of sarcopenia on surgical outcomes in non-small cell lung cancer. Ann Surg Oncol 27:2427–2435
Nakamura R, Inage Y, Tobita R et al (2018) Sarcopenia in resected NSCLC: effect on postoperative outcomes. J Thorac Oncol 13:895–903
Hervochon R, Bobbio A, Guinet C et al (2017) Body mass index and total Psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg 103:287–295
Suzuki Y, Okamoto T, Fujishita T et al (2016) Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer 101:92–97
Tsukioka T, Nishiyama N, Izumi N et al (2017) Sarcopenia is a novel poor prognostic factor in male patients with pathological Stage I non-small cell lung cancer. Jpn J Clin Oncol 47:363–368
Calle EE, Rodriguez C, Walker-Thurmond K et al (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625–1638
Gupta A, Majumder K, Arora N et al (2016) Premorbid body mass index and mortality in patients with lung cancer: a systematic review and meta-analysis. Lung Cancer 102:49–59
Lam VK, Bentzen SM, Mohindra P et al (2017) Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 104:52–57
Mitsuyoshi T, Matsuo Y, Itou H et al (2018) Evaluation of a prognostic scoring system based on the systemic inflammatory and nutritional status of patients with locally advanced non-small-cell lung cancer treated with chemoradiotherapy. J Radiat Res 59:50–57
Bowden JCS, Williams LJ, Simms A et al (2017) Prediction of 90 day and overall survival after chemoradiotherapy for lung cancer: role of performance status and body composition. Clin Oncol (R Coll Radiol) 29:576–584
Matsuo Y, Mitsuyoshi T, Shintani T et al (2018) Impact of low skeletal muscle mass on non-lung cancer mortality after stereotactic body radiotherapy for patients with stage I non-small cell lung cancer. J Geriatr Oncol 9:589–593
Kiss N, Beraldo J, Everitt S (2019) Early skeletal muscle loss in non-small cell lung cancer patients receiving chemoradiation and relationship to survival. Support Care Cancer 27:2657–2664
Fujiwara N, Nakagawa H, Kudo Y et al (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63:131–140
Okumura S, Kaido T, Hamaguchi Y et al (2017) Impact of skeletal muscle mass, muscle quality, and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann Surg Oncol 24:1037–1045
Katsui K, Ogata T, Watanabe K et al (2019) Dose-volume parameters predict radiation pneumonitis after induction chemoradiotherapy followed by surgery for non-small cell lung cancer: a retrospective analysis. BMC Cancer 19:1144
Segawa Y, Kiura K, Takigawa N et al (2010) Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol 28:3299–3306
Sato H, Toyooka S, Soh J et al (2016) The feasibility of median sternotomy with or without thoracotomy for locally advanced non-small cell lung cancer treated with induction chemoradiotherapy. Ann Thorac Surg 102:985–992
Katsui K, Ogata T, Tada A et al (2021) A PET/CT volumetric parameter predicts prognosis of non-small cell lung cancer treated using preoperative chemoradiotherapy and surgery: a retrospective case series study. Mol Clin Oncol 14:73
Hamaguchi Y, Kaido T, Okumura S et al (2016) Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 32:1200–1205
Heagerty PJ, Lumley T, Pepe MS (2000) Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 56:337–344
Cao Y (2007) Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117:2362–2368
Lutz CT, Quinn LS (2012) Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 4:535–546
Freese KE, Kokai L, Edwards RP et al (2015) Adipose-derived stems cells and their role in human cancer development, growth, progression, and metastasis: a systematic review. Cancer Res 75:1161–1168
Ibrahim MM (2010) Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11:11–18
Gould DW, Lahart I, Carmichael AR et al (2013) Cancer cachexia prevention via physical exercise: molecular mechanisms. J Cachexia Sarcopenia Muscle 4:111–124
Delrieu L, Pialoux V, Pérol O et al (2020) Feasibility and health benefits of an individualized physical activity intervention in women with metastatic breast cancer: intervention study. JMIR Mhealth Uhealth 8:e12306
Mayo NE, Feldman L, Scott S et al (2011) Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery 150:505–514
Acknowledgements
We are grateful to Dr. Shimpei Tsudaka and Dr. Hiromasa Yamamoto (Department of Thoracic Surgery, Okayama University Hospital) and Soichi Sugiyama (Department of Radiology, Okayama University Hospital) for data collection. We extend our sincere appreciation to all the patients who participated in this study.
Funding
This research was funded by the Tsuyama Chuo Hospital. The sponsors of this research were not involved in the conduct of this study, including study design, data collection, data analysis, data interpretation, manuscript writing, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
KK collected the data and drafted the manuscript; TO performed the statistical analysis; and KW collected the data. All authors participated in the design of the study and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest with respect to this study.
Ethical approval
The institutional review board of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and Okayama, University Hospital approved this study (approval number: 1809-018). The need for written informed consent was waived due to the retrospective nature of the study. The choice to opt out was provided through notifications displayed on the hospital’s website and outpatient ward before starting this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Katsui, K., Ogata, T., Watanabe, K. et al. Sarcopenia is related to poor prognosis in patients after trimodality therapy for locally advanced non-small cell lung cancer. Int J Clin Oncol 26, 1450–1460 (2021). https://doi.org/10.1007/s10147-021-01927-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01927-7