Abstract
In this study, we seek to explore the incidence of and potential risk factors for postoperative infarctions after meningioma surgery, in addition to the possible association with new neurological deficits, seizures, and health-related quality of life (HRQoL). A single-center cohort study was conducted, where all patients operated for an intracranial meningioma at our institution between 2007 and 2020 were screened for inclusion. Clinical data were prospectively collected in a local tumor registry, and HRQoL was assessed using both generic and disease-specific instruments. In total, 327 meningioma operations were included, and early postoperative MRIs showed peritumoral infarctions in 114 (34.9%). Median infarction volume was 4.5 ml (interquartile range 2.0–9.5) and 43 (37.7%) of the infarctions were rim-shaped, 44 (38.6%) were sector-shaped, 25 (21.9%) were a combination of rim- and sector-shaped, and two (1.8%) were remote infarctions. Permanent neurological deficits were seen in 22 patients (6.7%) and deficits were associated with infarctions (p < 0.001). There was no difference in frequency of registered postoperative epilepsy between patients with versus without infarctions. Patients with infarctions reported more future uncertainty; otherwise, there were no significant differences in disease specific HRQoL between patients with versus without infarctions. In this study, we found that peritumoral infarctions after meningioma resection are common. Most patients with permanent neurological deficits had infarctions. Yet, most infarctions were small, and although sometimes symptomatic on individual level, infarctions did not lead to significant deterioration of HRQoL on group level.
Similar content being viewed by others
Background
Meningioma is the most common primary intracranial tumor [6, 22], with a reported prevalence of 0.9% and 1.0% in population-based MRI studies [11, 33]. Patients with growing or symptomatic tumors are often offered surgery. Although patients operated for meningioma exhibit good tumor control and survival rates [17, 31, 35], potential benefits of surgery and the intraoperative choices must be balanced against the risk of new or worsened neurological deficits and postoperative complications.
Postoperative complications after meningioma surgery may be associated with decreased survival [14], and 4–14% experience neurological deterioration following surgery [2, 14, 24]. The risk of surgically induced epilepsy is 13–19% [15, 34], and emotional and cognitive difficulties following meningioma surgery have been reported in up to 40%, as well as frequent problems with postoperative fatigue [19, 32]. In Sweden, the proportion of patients working 2 years after meningioma surgery was only 57% compared to 84% in matched controls [29].
Although there is an increased awareness of the importance of peritumoral infarctions related to glioma surgery [1, 4, 8, 12, 23, 26, 28], peritumoral infarctions after meningioma resection are less studied and the main focus has been on venous infarctions [27]. However, a recent publication with 122 patients found that an area with MR restricted diffusion over 1 cm was seen after 22% of meningioma operations and was associated with postoperative deficits [16]. A recent study found that postoperative ischemia, defined as ischemia/infarction found on postoperative MRI not related to normal postoperative changes within the resection bed, occurred in 13 of 57 (23%) cases and was associated with postoperative seizures [7].
In this single-center prospective cohort study, we sought to study the incidence of and potential risk factors for postoperative infarctions after meningioma surgery as well as the possible association with new neurological deficits, seizures, and health-related quality of life (HRQoL).
Methods
Study cohort
We screened all adult patients (≥ 18 years) operated for intracranial meningioma at the Department of Neurosurgery at St. Olavs Hospital, Trondheim, Norway, from January 2007 through May 2020 with available postoperative MRI including DWI sequences taken less than 72 h after surgery. Patients with a postoperative CT-control only, with a postoperative MRI control done longer than 72 h after surgery, or without a histologically confirmed diagnosis were excluded.
Data collection
Patient characteristics were collected prospectively in a local tumor registry. Tumor grading was done by a neuropathologist. Meningiomas operated before the last half of 2016 were classified according to the WHO 2007 classification, and the WHO 2016 classification was used thereafter. Preoperative tumor volumes were segmented semi-automatically from pre-treatment MRIs. Surgical extent of resection was classified from postoperative MRI scans into gross-total resection (radiologically total tumor resection on early postoperative MRI) or subtotal resection (tumor remnants seen on early postoperative MRI). Starting in 2015, operating surgeons filled out a questionnaire immediately after surgery where they reported on intraoperative tumor characteristics. Neurological deficits were defined as either a permanent paresis or permanent dysphasia/aphasia registered at follow-ups 4 weeks or longer after surgery and were retrospectively retrieved from electronical medical records.
Our postoperative MRI protocols and routines for segmentation of infarctions and tumors have been described in detail earlier [26]. Briefly, infarctions were detected using postoperative B100 sequences on diffusion-weighted images (DWI) and apparent diffusion coefficient (ADC) maps. Areas with high signals on B1000 images and corresponding low values on ADC maps, which could not be explained by other diffusion abnormalities, and with a thickness of at least 3 mm were defined as infarctions. Infarctions were classified as either rim-shaped, sector-shaped, remote, or a combination of sector- and rim-shaped. Examples of sector-shaped and rim-shaped infarctions are presented in Fig. 1. All infarctions were manually segmented using DWI, whereas tumors were segmented semi-automatically using preoperative T1-weighted images with contrast.
Following 112 of the operations from 2015 and onwards, surgeons reported on a questionnaire immediately after surgery whether or not there had been a certain or probable intraoperative injury to a functional artery during surgery.
Quality of life
One to 3 days before surgery, the patients completed HRQOL questionnaires on. In cases where the patient was unable to complete the questionnaire (for instance because of cognitive impairment or language deficits), a study nurse or a next of kin helped. Such proxy ratings in neuro-oncological patients have shown good agreement with the patients’ own scores [9, 25]. A 1-month follow-up of HRQoL was done as a structured telephone interview conducted by a study nurse.
EQ-5D 3L is a generic questionnaire designed by the EuroQol Group and consists of a descriptive system and a visual analog scale (VAS) [5]. The descriptive system covers five dimensions, mobility, self-care, activity, pain, and anxiety, scored as either “no problem,” “slight problem,” or “major problem.” EQ-5D 3L is used for numerous conditions and has been validated for the Norwegian population [13, 20]. One patient had four missing domains in the preoperative questionnaire and was excluded. Six patients had one missing item on preoperative questionnaires, five of these were imputed as “no problem” as they reported “no problem” in all but one missing domain, whereas one responder reported “slight problem” in all but one missing domain and in this case “slight problem” was imputed. Two patients had one missing item on postoperative questionnaires, and both were imputed as “no problem” as they reported “no problem” in all other domains.
EORTC QLQ-C30 is a frequently used disease-specific questionnaire to score HRQoL in cancer patients and is used to assess symptoms and functional outcomes for many cancers [10, 37]. It covers central domains in daily life, for instance physical, emotional, and role functioning, in addition to symptoms common to all cancers, such as pain, nausea, fatigue, and loss of appetite.
EORTC BN20 is a supplement for patients with brain cancer, to be used in combination with QLQ-30 [21]. It compromises four multi-item scales of future uncertainty, visual impairment, motor dysfunction, and communication problems. In addition, there are seven single-item scales on symptoms related to brain cancers. Data from EORTC QLQ-C30 and BN20 were only available from 2015 and onwards, and no imputations for missing data were done for these disease-specific instruments.
Statistical analyses
Statistical analyses and descriptive statistics were performed with IBS SPSS Statistics version 27.0 (IBM, Armonk, NY). Q-Q plots were used to assess normal distribution. A binominal logistic regression model was constructed to establish risk factors for infarctions, and for these analyses included variables were chosen based on presumed relevance. Fisher’s exact tests were applied for calculations of neurological deficits due to a small number of events. Statistical significance level was set to p ≤ 0.05.
Ethics and approval
All patients provided written informed consent to be included in research, and the study was approved by the regional ethics committee (REK) (reference 2018/1187). Data collection was done according to the guidelines of the Helsinki Declaration.
Results
We identified 422 patients with meningiomas who underwent 467 operations, of which 327 (70.2%) had an early postoperative MRI, 118 (25.3%) only had a postoperative CT scan, and 22 (4.7%) underwent no early postoperative imaging control.
Among the 327 meningioma operations with early MRI scans, 114 (34.9%) showed peritumoral infarctions. Median total infarction volume was 4.5 ml (interquartile range 2.0–9.5). Forty-three (37.7%) of the infarctions were rim-shaped, 44 (38.6%) were sector-shaped, 25 (21.9%) were a combination of rim- and sector-shaped, and two (1.8%) were remote infarctions. Median infarction volumes in different subgroups were 2.3 cm3 (interquartile range [IQR] 1.3–3.1), 8.3 cm3 (IQR 4.3–13.2), 8.2 cm3 (IQR 3.5–10.7), and 0.27 cm3 for rim, sector, combined sector and rim, and remote infarctions, respectively. Postoperative infarctions were more common is surgeons reported intraoperative damage to a functional artery, 17 of 38 (44.7%) vs 12 of 74 (16.2%), p = 0.003.
A binominal logistic regression that predicts the likelihood of postoperative infarctions is presented in Table 1. As seen, increasing age is associated with an increased likelihood of postoperative infarctions, and there was a non-significant trend that larger tumors were associated with postoperative infarctions.
New neurological deficits
Acquired language or motor deficits are presented in Table 2. As seen, neurological sequelae were seen in 22 patients (6.7%) and were more common in patients with infarctions. Among 6 patients without infarctions who still experienced permanent deficits after surgery, one patient had a postoperative hematoma that required urgent surgery, and five remaining patients had parasagittal meningiomas affecting the primary motor cortex and likely had direct damage to the underlying primary motor cortex during dissection. There was no difference in frequency of registered postoperative epilepsy between patients with versus without infarctions, 5.1% vs 3.6 (p = 0.549), respectively.
Quality of life
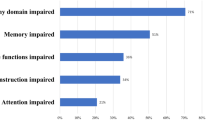
In total, 227 and 225 completed the EQ-5D questionnaires before and after surgery, respectively. Patients reported an overall better quality of life after surgery, with a median index value at 0.796 before surgery (IQR 0.689–0.848) and 0.812 one month after surgery (IQR 0.714–1.00), p = 0.001. There was no significant difference in change in EQ5D-index value 1 month after surgery in patients with versus without infarctions (median change 0.052 (IQR − 0.071–0.20) vs 0.0 (IQR − 0.36–0.16), p = 0.435). In Fig. 2, the distributions of change for each dimension in EQ5D from before and to 1 month after surgery are presented. As seen, there were no significant differences between patients with versus without infarctions in the five EQ5D domains, and most patients reported no change in mobility, self-care, pain, anxiety, or activity 1 month after surgery.
For the EORTC QLQ-C30 and EORTC QLQ-BN20 instruments, 141 patients completed the questionnaires. Patients with infarctions reported more future uncertainty (median change from baseline 16.7 (IQR 8.3–41.6) vs 8.3 (0–41.6), p = 0.048). Otherwise, there was no statistical difference in the functional or symptom scales when comparing patients with and without infarctions.
Discussion
In this single-center population-based cohort study, we found that brain infarctions are common after meningioma surgery and are associated with higher risk of permanent neurological deficits. However, most infarctions are functionally silent and there is no significant effect on patient-reported HRQoL on group level. Patients with postoperative infarctions were older than patients without infarctions.
The risk of infarctions following meningioma surgery is high, and mean infarction and mean infarction volumes are even larger than for glioma operations [22]. The vascular supply of the aging brain is more vulnerable and, like in glioma surgery, the risk of infarctions is higher with age [22]. Many meningiomas involve underlying cortical vessels, and may even engulf larger arteries, and damage to both arteries and veins may occur inadvertently and may sometimes be unavoidable during the dissection. Also, sometimes vascular supply of meningiomas may in part be shared by the cortex and the tumor, adding to the risk of resection. In larger tumors, more vessels are often at risk and visual control of vessels may be poorer if the tumor is resected en bloc. Larger meningiomas tend to recruit more blood supply from the underlying brain, and visual control of underlying normal vessels can be difficult even after extensive debulking, especially in firmer tumors. Thus, as perhaps expected, we found a trend that risk of infarctions was larger in larger tumors. However, in contrast to our findings, a recent study found that tumor size was not associated with significant infarctions, defined as DWI signal larger than 1 cm [16]. This might be explained by a smaller sample size and that only convexity meningiomas were included in that study. Nevertheless, as pointed out by the authors, how adherent a meningioma is to cerebral cortex might be more challenging than the size of the meningioma itself. Still, we did not find a significant lower risk of infarctions in WHO grade 1 meningiomas that per definition do not infiltrate normal tissue and more seldom exhibit peritumoral edema.
The risk of infarctions is naturally higher, but not 100%, if the surgeon reported sacrifice of an assumed normal artery, reflecting that collateral blood supply can be unpredictable. Surgeons should strive to develop dissection techniques that limit the risk of infarctions. In the present study, we did not assess the potential effect of different surgical techniques and tools, yet these factors may impact the likelihood of postoperative infarctions. Increasing awareness among neurosurgeons and neuroradiologists of the importance of DWI changes on early postoperative MRIs and inclusion of these sequences in routine assessments may in itself be a step forward towards developing better and safer surgery, and individual surgeons should attempt to learn from their postoperative MRI findings to make potential adjustments in their technique. In vulnerable regions, or in tumors that engulf important vessels or are very adherent, subtotal resections and/or stereotactic radiosurgery may be a viable alternative.
Most patients operated for meningioma have good survival rates and most meningiomas are slow-growing tumors. Although the potential for rehabilitation is larger with increased survival, weighing potential benefits of tumor resection against risk of surgically induced permanent deficits is important in preoperative planning, and the risk of peritumoral infarctions should add to the preoperative evaluation of surgery-related risks. Although both definitions of deficits and time of assessment vary across studies, the risk of neurological deficits following meningioma surgery is significant. In a Swedish national registry-based study, 14.8% developed new neurologic deficits following meningioma surgery while 8.3% of preoperative asymptomatic patients developed deficits [3]. A large Norwegian population-based cohort study found that new postoperative neurological deficits occur in only 4% of meningioma resections, and that postoperative hematoma was the only significant predictor for postoperative neurological deterioration [14]. Although postoperative hematomas can lead to new sequelae, the present study indicates that peritumoral infarctions may be an important and perhaps less acknowledged cause of postoperative deficits. As seen, the majority of patients with acquired postoperative deficits have surgery-related infarctions.
Still, some patients acquired neurological deficits without infarctions or hematomas, and the second most common cause of new deficits may be direct cortical damage during dissection. However, the sensitivity of DWI sequences for detecting very small infarctions may be low as the slice thickness is often several millimeters, and artifacts, for example, from clamps and plates used for bone flap fixation or small hematomas, may hamper interpretation. In the present study, we used a 3-mm cut for significant infarctions, as done in earlier studies [12, 26], yet smaller infarctions may also cause deficits if located in eloquent areas.
Unlike a recent study [7], we did not find an association between infarctions and risk of postoperative epilepsy. However, we did not account for preoperative seizures in the analyses and with longer follow-ups frequencies of seizures would be expected to increase. In a Swedish registry-based study, nearly one in five meningioma patients were still on antiepileptic drugs 2 years after surgery [30]. Thus, due to the frequent use of antiepileptic drugs postoperatively in patients with preoperative seizures, and due to the short follow-up in many studies, underestimation of postoperative seizure frequencies is likely.
In the present study, no difference in HRQoL was found between patients with versus without infarctions. HRQoL in patients harboring meningiomas is not much studied, but a systematic review found that patients reported worse HRQoL than healthy controls both prior to and after surgery [36]. The observed lack of a difference in complete case analysis of HRQoL in the two groups in our study might be influenced by the fact that data on HRQoL may not be missing at random, and may also reflect that the questionnaires used do not necessarily cover all important aspects of quality of life in patients with meningiomas [18]. Moreover, patients with larger tumors more often had infarctions, and larger tumors may have a greater preoperative impact on HRQoL, thus masquerading the true effects of peritumoral infarctions.
This study has some other limitations. As patients operated for meningiomas have good survival rates, prospective long-time data on neurological sequelae because of peritumoral infarctions would be of interest, as even permanent deficits may get better after rehabilitation. In addition, EORTC QLQ-C30 and BN20 were not collected in the entire study period, and a larger sample size with completed questionnaires might have yielded different results. Further, revisions of the WHO classification system during the inclusion period may have affected the number of patients with atypical meningiomas in the study. Strengths in this study include patient-reported measurements of quality of life, the relatively large sample size, manual segmentations of all tumors and infarctions for precise volumetric assessment, and prospective collection of clinical data.
Conclusion
In this prospective single-center cohort study, we found that one third of the patients operated for an intracranial meningioma had infarctions detected by early postoperative MRIs. Infarctions were more common in older patients, and most patients with permanent neurological deficits had infarctions. However, most infarctions were rather small and on group level, infarctions did not lead to significant deterioration of HRQoL.
Code availability
Not applicable.
References
Bette S, Barz M, Huber T, Straube C, Schmidt-Graf F, Combs SE, Delbridge C, Gerhardt J, Zimmer C, Meyer B, Kirschke JS, Boeckh-Behrens T, Wiestler B, Gempt J (2018) Retrospective analysis of radiological recurrence patterns in glioblastoma, their prognostic value and association to postoperative infarct volume. Sci Rep 8:4561. https://doi.org/10.1038/s41598-018-22697-9
Chan RC, Thompson GB (1984) Morbidity, mortality, and quality of life following surgery for intracranial meningiomas: a retrospective study in 257 cases. J Neurosurg 60:52–60
Corell A, Thurin E, Skoglund T, Farahmand D, Henriksson R, Rydenhag B, Gulati S, Bartek J, Jakola AS (2019) Neurosurgical treatment and outcome patterns of meningioma in Sweden: a nationwide registry-based study. Acta Neurochir (Wien) 161:333–341
Dutzmann S, Gessler F, Bink A, Quick J, Franz K, Seifert V, Senft C (2012) Risk of ischemia in glioma surgery: comparison of first and repeat procedures. J Neurooncol 107:599–607. https://doi.org/10.1007/s11060-011-0784-1
EuroQol G (1990) EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 16:199–208. https://doi.org/10.1016/0168-8510(90)90421-9
Fathi A-R, Roelcke U (2013) Meningioma. Curr Neurol Neurosci Rep 13:337
Gadot R, Khan AB, Patel R, Goethe E, Shetty A, Hadley CC, Harmanci AS, Klisch TJ, Yoshor D, Sheth SA (2021) Predictors of postoperative seizure outcome in supratentorial meningioma. J Neurosurg 1:1–10
Gempt J, Forschler A, Buchmann N, Pape H, Ryang YM, Krieg SM, Zimmer C, Meyer B, Ringel F (2013) Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J Neurosurg 118:801–808. https://doi.org/10.3171/2012.12.JNS12125
Giesinger JM, Golser M, Erharter A, Kemmler G, Schauer-Maurer G, Stockhammer G, Muigg A, Hutterer M, Rumpold G, Holzner B (2009) Do neurooncological patients and their significant others agree on quality of life ratings? Health Qual Life Outcomes 7:87. https://doi.org/10.1186/1477-7525-7-87
Giesinger JM, Kuijpers W, Young T, Tomaszewski KA, Friend E, Zabernigg A, Holzner B, Aaronson NK (2016) Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain. Health Qual Life Outcomes 14:87. https://doi.org/10.1186/s12955-016-0489-4
Håberg AK, Hammer TA, Kvistad KA, Rydland J, Müller TB, Eikenes L, Gårseth M, Stovner LJ (2016) Incidental intracranial findings and their clinical impact; the HUNT MRI study in a general population of 1006 participants between 50–66 years. PLoS ONE 11:e0151080
Jakola AS, Berntsen EM, Christensen P, Gulati S, Unsgard G, Kvistad KA, Solheim O (2014) Surgically acquired deficits and diffusion weighted MRI changes after glioma resection—a matched case-control study with blinded neuroradiological assessment. PLoS ONE 9:e101805. https://doi.org/10.1371/journal.pone.0101805
Jakola AS, Unsgard G, Solheim O (2011) Quality of life in patients with intracranial gliomas: the impact of modern image-guided surgery. J Neurosurg 114:1622–1630. https://doi.org/10.3171/2011.1.JNS101657
Lemée J-M, Corniola MV, Da Broi M, Schaller K, Meling TR (2019) Early postoperative complications in meningioma: predictive factors and impact on outcome. World Neurosurg 128:e851–e858
Li X, Wang C, Lin Z, Zhao M, Ren X, Zhang X, Jiang Z (2020) Risk factors and control of seizures in 778 Chinese patients undergoing initial resection of supratentorial meningiomas. Neurosurg Rev 43:597–608
Magill ST, Nguyen MP, Aghi MK, Theodosopoulos PV, Villanueva-Meyer JE, McDermott MW (2021) Postoperative diffusion-weighted imaging and neurological outcome after convexity meningioma resection. J Neurosurg 1:1–8
McCarthy BJ, Davis FG, Freels S, Surawicz TS, Damek DM, Grutsch J, Menck HR, Laws ER (1998) Factors associated with survival in patients with meningioma. J Neurosurg 88:831. https://doi.org/10.3171/jns.1998.88.5.0831
Najafabadi AHZ, Peeters MC, Lobatto DJ, Broekman ML, Smith TR, Biermasz NR, Peerdeman SM, Peul WC, Taphoorn MJ, van Furth WR (2017) Health-related quality of life of cranial WHO grade I meningioma patients: are current questionnaires relevant? Acta Neurochir (Wien) 159:2149–2159
Nassiri F, Price B, Shehab A, Au K, Cusimano MD, Jenkinson MD, Jungk C, Mansouri A, Santarius T, Suppiah S (2019) Life after surgical resection of a meningioma: a prospective cross-sectional study evaluating health-related quality of life. Neuro Oncol 21:i32–i43
Nord E (1991) EuroQol: health-related quality of life measurement. Valuations of health states by the general public in Norway. Health Policy 18:25–36. https://doi.org/10.1016/0168-8510(91)90141-j
Osoba D, Aaronson NK, Muller M, Sneeuw K, Hsu MA, Yung WK, Brada M, Newlands E (1996) The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res 5:139–150. https://doi.org/10.1007/BF00435979
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17(Suppl 4):iv1–iv62. https://doi.org/10.1093/neuonc/nov189
Sales AHA, Barz M, Bette S, Wiestler B, Ryang YM, Meyer B, Bretschneider M, Ringel F, Gempt J (2017) Impact of ischemic preconditioning on surgical treatment of brain tumors: a single-center, randomized, double-blind, controlled trial. BMC Med 15:137. https://doi.org/10.1186/s12916-017-0898-1
Sicking J, Voß KM, Spille DC, Schipmann S, Holling M, Paulus W, Hess K, Steinbicker AU, Stummer W, Grauer O (2018) The evolution of cranial meningioma surgery—a single-center 25-year experience. Acta Neurochir (Wien) 160:1801–1812
Sneeuw KC, Aaronson NK, Osoba D, Muller MJ, Hsu MA, Yung WK, Brada M, Newlands ES (1997) The use of significant others as proxy raters of the quality of life of patients with brain cancer. Med Care 35:490–506. https://doi.org/10.1097/00005650-199705000-00006
Strand PS, Berntsen EM, Fyllingen EH, Sagberg LM, Reinertsen I, Gulati S, Bouget D, Solheim O (2021) Brain infarctions after glioma surgery: prevalence, radiological characteristics and risk factors. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-021-04914-z
Sughrue ME, Rutkowski MJ, Shangari G, Fang S, Parsa AT, Berger MS, McDermott MW (2011) Incidence, risk factors, and outcome of venous infarction after meningioma surgery in 705 patients. J Clin Neurosci 18:628–632
Thiepold AL, Luger S, Wagner M, Filmann N, Ronellenfitsch MW, Harter PN, Braczynski AK, Dutzmann S, Hattingen E, Steinbach JP, Senft C, Rieger J, Bahr O (2015) Perioperative cerebral ischemia promote infiltrative recurrence in glioblastoma. Oncotarget 6:14537–14544. https://doi.org/10.18632/oncotarget.3994
Thurin E, Corell A, Gulati S, Smits A, Henriksson R, Bartek J Jr, Salvesen Ø, Jakola AS (2020) Return to work following meningioma surgery: a Swedish nationwide registry-based matched cohort study. Neuro-oncology practice 7:320–328
Thurin E, Rydén I, Skoglund T, Smits A, Gulati S, Hesselager G, Bartek J Jr, Henriksson R, Salvesen Ø, Jakola AS (2021) Impact of meningioma surgery on use of antiepileptic, antidepressant, and sedative drugs: a Swedish nationwide matched cohort study. Cancer Med 10:2967–2977
van Alkemade H, de Leau M, Dieleman EM, Kardaun JW, van Os R, Vandertop WP, van Furth WR, Stalpers LJ (2012) Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol 14:658–666
van der Vossen S, Schepers VP, van der Sprenkel JWB, Visser-Meily J, Post MW (2014) Cognitive and emotional problems in patients after cerebral meningioma surgery. J Rehabil Med 46:430–437
Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357:1821–1828
Wirsching H-G, Morel C, Gmür C, Neidert MC, Baumann CR, Valavanis A, Rushing EJ, Krayenbühl N, Weller M (2016) Predicting outcome of epilepsy after meningioma resection. Neuro Oncol 18:1002–1010
Waagemans ML, van Nieuwenhuizen D, Dijkstra M, Wumkes M, Dirven CMF, Leenstra S, Reijneveld JC, Klein M, Stalpers LJA (2011) Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery 69:72–79. https://doi.org/10.1227/NEU.0b013e318212badb
Zamanipoor Najafabadi AH, Peeters MCM, Dirven L, Lobatto DJ, Groen JL, Broekman MLD, Peerdeman SM, Peul WC, Taphoorn MJB, van Furth WR (2016) Impaired health-related quality of life in meningioma patients—a systematic review. Neuro Oncol 19:897–907. https://doi.org/10.1093/neuonc/now250
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376. https://doi.org/10.1093/jnci/85.5.365
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital) The study was supported by the Norwegian Research Council and the Faculty of Medicine at the Norwegian University of Science and Technology (NTNU), through the Student Research Program at NTNU. The funders did not take part in the study design, data collection, data analysis, publishing process, or review of the paper.
Author information
Authors and Affiliations
Contributions
PSS: data acquisition, writing of original draft, statistical analyses. SG: conceptualization, data acquisition, supervision. LMS: data acquisition, resources. OS: conceptualization, data acquisition, supervision, writing. All authors contributed to and reviewed the final manuscript.
Corresponding author
Ethics declarations
Ethical considerations
All patients provided written informed consent to be included in research, and the study was approved by the regional ethics committee (REK) (reference 2018/1187). Data collection was done according to the guidelines of the Helsinki Declaration.
Consent to participate
Written, informed consent to participate in research was acquired from all patients or a next-of-kin.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The paper has not been published or presented previously.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strand, P.S., Sagberg, L.M., Gulati, S. et al. Brain infarction following meningioma surgery—incidence, risk factors, and impact on function, seizure risk, and patient-reported quality of life. Neurosurg Rev 45, 3237–3244 (2022). https://doi.org/10.1007/s10143-022-01840-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-022-01840-1