Abstract
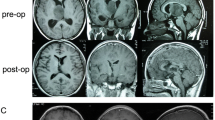
This study aimed to identify (1) the thalamic gliomas suitable for surgical resection and (2) the appropriate surgical approach based on their location and the displacement of the posterior limb of the internal capsule (PLIC). A retrospective study over a 5-year period (from 2006 to 2010) was performed in 41 patients with thalamic gliomas. The mean age of these patients was 20.4 years (range, 2–65 years). Twenty (49 %) tumors were thalamic, 19 (46 %) were thalamopeduncular, and 2 (5 %) were bilateral. The PLIC, based on T2-weighted magnetic resonance axial sections, was displaced anterolaterally in 23 (56 %) cases and laterally in 6 (14 %) cases. It was involved by lesion in eight (20 %) cases and could not be identified in four (10 %) cases. Resection, favored in patients with well-defined, contrast-enhancing lesions, was performed in 34 (83 %) cases, while a biopsy was resorted to in 7 (17 %) cases. A gross total resection or near total resection (>90 %) could be achieved in 26 (63 %) cases. The middle temporal gyrus approach, used when the PLIC was displaced anterolaterally, was the commonly used approach (63.5 %). Common pathologies were pilocytic astrocytoma (58 %) in children and grade III/IV astrocytomas (86 %) in adults. Preoperative motor deficits improved in 64 % of the patients with pilocytic lesions as compared to 0 % in patients with grade III/IV lesions (P value, 0.001). Postoperatively, two patients (5 %) had marginal worsening of motor power, two patients developed visual field defects, and one patient developed a third nerve paresis. Radical resection of thalamic gliomas is a useful treatment modality in a select subset of patients and is the treatment of choice for pilocytic astrocytomas. Tailoring the surgical approach, depending on the relative position of the PLIC, has an important bearing on outcome.






Similar content being viewed by others
References
Albright AL (2004) Feasibility and advisability of resections of thalamic tumors in pediatric patients. J Neurosurg pediatr 100:468–472
Arseni C (1958) Tumors of the basal ganglia. Their surgical treatment. Arch Neurol Psychiatry 80:18–24
Beks JWF, Bouma GJ, Journée HL (1987) Tumours of the thalamic region. A retrospective study of 27 cases. Acta Neurochir 85:125–127
Berman JI, Berger MS, Mukherjee P, Henry RG (2004) Diffusion-tensor imaging-guided tracking of fibers of the pyramidal tract combined with intraoperative cortical stimulation mapping in patients with gliomas. J Neurosurg 101:66–72
Bernstein M, Hoffman HJ, Halliday WC, Hendrick EB, Humphreys RP (1984) Thalamic tumors in children. Long-term follow-up and treatment guidelines. J Neurosurg 61:649–656
Broadway SJ, Ogg RJ, Scoggins MA, Sanford R, Patay Z, Boop FA (2011) Surgical management of tumors producing the thalamopeduncular syndrome of childhood. J Neurosurg Pediatr 7:589–595
Cheek WR, Taveras JM (1966) Thalamic tumors. J Neurosurg 24:505–513
Cuccia V, Monges J (1997) Thalamic tumors in children. Childs Nerv Syst 13:514–521
D’Angelo VA, Galarza M, Catapano D, Monte V, Bisceglia M, Carosi I (2005) Lateral ventricle tumors: surgical strategies according to tumor origin and development—a series of 72 cases. Neurosurgery 56(1 Suppl):36–45
Di Rocco C, Iannelli A (2002) Bilateral thalamic tumors in children. Childs Nerv Syst 18:440–444
Drake JM, Joy M, Goldenberg A, Kreindler D (1991) Computer- and robot-assisted resection of thalamic astrocytomas in children. Neurosurgery 29:27–33
Egan RA, Shults WT, So N, Burchiel K, Kellogg JX, Salinsky M (2000) Visual field deficits in conventional anterior temporal lobectomy versus amygdalohippocampectomy. Neurology 55:1818–1822
El Beltagy MA, Aggag M, Kamal M (2010) Role of intraoperative ultrasound in resection of pediatric brain tumors. Childs Nerv Syst 26:1181–1193
Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz Paredes A et al (2003) Pilocytic astrocytomas in children: prognostic factors—a retrospective study of 80 cases. Neurosurgery 53:544–555
Frank F, Fabrizi AP, Gaist G, Frank-Ricci R, Piazzi M, Spagnolli F (1987) Stereotaxy and thalamic masses. Survey of 44 cases. Appl Neurophysiol 50:243–247
Franzini A, Leocata F, Cajola L, Servello D, Allegranza A, Broggi G (1994) Low-grade glial tumors in basal ganglia and thalamus: natural history and biological reappraisal. Neurosurgery 35:817–820
Gottfried ON, Fults DW, Townsend JJ, Couldwell WT (2003) Spontaneous hemorrhage associated with a pilomyxoid astrocytoma. Case report. J Neurosurg 99:416–420
Greenwood J Jr (1973) Radical surgery of tumors of the thalamus, hypothalamus, and third ventricle area. Surg Neurol 1:29–33
Hamada H, Kurimoto M, Hayashi N, Nagai S, Kurosaki K, Nomoto K et al (2008) Pilomyxoid astrocytoma in a patient presenting with fatal hemorrhage. Case report. Journal of Neurosurg Pediatr 1:244–246
Hoffman HJ, Soloniuk DS, Humphreys RP, Drake JM, Becker LE, De Lima BO et al (1993) Management and outcome of low-grade astrocytomas of the midline in children: a retrospective review. Neurosurgery 33:964–971
Kamada K, Todo T, Masutani Y, Aoki S, Ino K, Takano T et al (2005) Combined use of tractography-integrated functional neuronavigation and direct fiber stimulation. J Neurosurg 102:664–672
Kelly PJ (1989) Stereotactic biopsy and resection of thalamic astrocytomas. Neurosurgery 25:185–195
Komotar RJ, Burger PC, Carson BS, Brem H, Olivi A, Goldthwaite PT et al (2004) Pilocytic and pilomyxoid hypothalamic/chiasmatic astrocytomas. Neurosurgery 54:72–80
Krouwer HG, Prados MD (1995) Infiltrative astrocytomas of the thalamus. J Neurosurg 82:548–557
Lyons MK, Kelly PJ (1992) Computer-assisted stereotactic biopsy and volumetric resection of thalamic pilocytic astrocytomas. Report of 23 cases. Stereotact Funct Neurosurg 59:100–104
Martinez-Lage JF, Perez-Espejo MA, Esteban JA, Poza M (2002) Thalamic tumors: clinical presentation. Childs Nerv Syst 18:405–411
Mc Girr SJ, Kelly PJ, Scheithauer BW (1987) Stereotactic resection of juvenile pilocytic astrocytomas of thalamus and basal ganglia. Neurosurgery 20:447–452
McKissock W, Paine KWE (1958) Primary tumours of the thalamus. Brain 81:41–63
Mengesha T, Abu-Ata M, Haas KF, Lavin PJ, Sun DA, Konrad PE et al (2009) Visual field defects after selective amygdalohippocampectomy and standard temporal lobectomy. J Neuroophthalmol 29:208–213
Moshel YA, Elliott RE, Monoky DJ, Wisoff JH (2009) Role of diffusion tensor imaging in resection of thalamic juvenile pilocytic astrocytoma. J Neurosurg Pediatr 4:495–505
Moshel YA, Link MJ, Kelly PJ (2007) Stereotactic volumetric resection of thalamic pilocytic astrocytomas. Neurosurgery 61:66–75
Nayar VV, Foroozan R, Weinberg JS, Yoshor D (2009) Preservation of visual fields with the inferior temporal gyrus approach to the atrium. J Neurosurg 110:740–743
Nishio S, Morioka T, Suzuki S, Takeshita I, Fukui M (1997) Thalamic gliomas: a clinicopathologic analysis of 20 cases with reference to patient age. Acta Neurochir 139:336–342
Ozek MM, Ture U (2002) Surgical approach to thalamic tumors Childs Nerv Syst 18:450–456
Partlow GD, del Carpio-O’Donovan R, Melanson D, Peters TM (1992) Bilateral thalamic glioma: review of eight cases with personality change and mental deterioration. Am J Neuroradiol 13:1225–1230
Puget S, Crimmins DW, Garnett MR, Grill J, Oliveira R, Boddaert N et al (2007) Thalamic tumors in children: a reappraisal. J Neurosurg pediatr 106:354–362
Reardon DA, Gajjar A, Sanford RA, Heideman RL, Walter AW, Thompson SJ et al (1998) Bithalamic involvement predicts poor outcome among children with thalamic glial tumors. Pediatr Neurosurg 29:29–35
Renowden SA, Matkovic Z, Adams CB, Carpenter K, Oxbury S, Molyneux AJ et al (1995) Selective amygdalohippocampectomy for hippocampal sclerosis: postoperative MR appearance. Am J Neuroradiol 16:1855–1861
Steiger HJ, Gotz C, Schmid-Elsaesser R, Stummer W (2000) Thalamic astrocytomas: surgical anatomy and results of a pilot series using maximum microsurgical removal. Acta Neurochir 142:1327–1336
Thudium MO, Campos AR, Urbach H, Clusmann H (2010) The basal temporal approach for mesial temporal surgery: sparing the Meyer loop with navigated diffusion tensor tractography. Neurosurgery 67(2 Suppl Operative):385–390
Tomita T, Cortes RF (2002) Astrocytomas of the cerebral peduncle in children: surgical experience in seven patients. Childs Nerv Syst 18:225–230
Tovi D, Schisano G, Liljeqvist B (1961) Primary tumors of the region of the thalamus. J Neurosurg 18:730–740
Unsgaard G, Gronningsaeter A, Ommedal S, Nagelhus Hernes TA (2002) Brain operations guided by real-time two-dimensional ultrasound: new possibilities as a result of improved image quality. Neurosurgery 51:402–412
Villarejo F, Amaya C, Perez Diaz C, Pascual A, Alvarez Sastre C, Goyenechea F (1994) Radical surgery of thalamic tumors in children. Childs Nerv Syst 10:111–114
Woo SY, Donaldson SS, Cox RS (1988) Astrocytoma in children: 14 years’ experience at Stanford University Medical Center. J Clin Oncol 6:1001–1007
Yasargil MG (1996) Microneurosurgery of CNS tumors, IVB. Thieme, New York, pp 299–300
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Hussam Metwali, Hannover, Germany
Thalamic glioma is quite a difficult and challenging entity of supratentorial gliomas. Because of its deep location, the surgeon has to go through and circumvent many vital structures. In this article, the authors described their experience with 41 cases of thalamic gliomas with different grades. The selection of the surgical approach was based on the location of the internal capsule and the tumor extension. The authors have also adapted the middle temporal gyrus approach to access the majority of the cases due to the anterolateral displacement of the posterior limb of the internal capsule. The authors have also mentioned the other approaches they used.
On the other side, our practice is different. Thalamic glioma constitutes a good example of functional glioma surgery, in which not only tumor resection but also preservation of function is our aim. Apart from the conventional anatomical MRI, come the preoperative DTI with color orientation maps and fiber tracking as well as the functional MRI in consideration. The choice of the appropriate surgical approach in our institute depends on the concordance of many variables including the tumor extension, the location of the tumor in the thalamus, the location of the pyramidal tracts, the location of the optic radiation, the location of the fornix and its insertion point into the corpus callosum, the location of the venous point (point of joining of the thalamostriate vein and the septal vein into the great cerebral veins), the anterior communicating artery complex and dominance, tentorial steep and the location of vein of Galen, and cerebral dominance and location of the speech areas. We use, depending the previously mentioned variables, the transcallosal approaches (ipsilateral or contralateral), the translamina terminalis approach, the pterional approach, the transsulcal approach (ipsilateral or contralateral), the supracerebellar infratentorial approach, and the occipital transtentorial approach. Since 2007, surgery of thalamic gliomas is usually performed under intraoperative MRI control. It allows us to access the amount of resection. It also provides the possibility of new fiber tracking and readjusting of the neuronavigator accordingly. The surgery is also performed under continuous monitoring of the somatosensory evoked potentials and the motor evoked potential (MEP). Surgery could be also assisted with 5-ALA, 5-ALA endoscopy, or conventional endoscopy.
Identification of the pyramidal tract in case of edema is not a big problem. The point here is to analyze and understand the color orientation maps and to compare them to the constructed fiber tracts. This improves the accuracy of fiber tracking. Infiltrated or destructed tracts can be predicted clinically or by MEP. Color orientation maps are also very helpful.
The middle temporal gyrus approach to thalamic glioma is a not commonly used approach in our practice. It can cause direct trauma to the visual tract as well as injury of the speech center in the dominant hemisphere.
This difference in practice and the availability of technology are not negative issues in the presented article. The authors have honestly described their methodology and its limitations. The authors have invested a lot of time and effort to establish this work. Congratulations.
Walter Stummer, Münster, Germany
The authors present an impressive series of patients operated on for thalamopeduncular tumors. They carefully analyze the location of the posterior limb of the internal capsule and identify those patients with anterior or anterolateral displacement of this structure as suitable candidates for the approach used in the majority of their cases through the middle temporal gyrus. Their reported results in these demanding tumors are intriguing.
This work nicely expands the past experience of surgery for these challenging lesions and presents an additional option for individual planning of an appropriate surgical strategy. It must be remembered, however, that other approaches may be feasible, depending on individual anatomic considerations.
The authors use intraoperative ultrasound as a method for intraoperative tumor location and for assessment of radicality. They do not use neuronavigation, nor do they integrate tractography for identification of the optical tract or the pyramidal tract, respectively. They also do not use subcortical stimulation or language mapping in left temporal approaches, not out of conviction but due to limitations of their resources. Such technology would serve to further enhance safety and radicality of surgery in this sensitive area. Nevertheless, the authors demonstrate good results in difficult tumors to be possible even if technology is not perfect.
Rights and permissions
About this article
Cite this article
Sai Kiran, N.A., Thakar, S., Dadlani, R. et al. Surgical management of thalamic gliomas: case selection, technical considerations, and review of literature. Neurosurg Rev 36, 383–393 (2013). https://doi.org/10.1007/s10143-013-0452-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-013-0452-3