Abstract
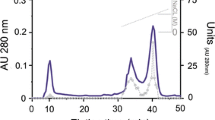
High activity of carboxypeptidases was detected in the hepatopancreas of the crab Paralithodes camtschatica, while aminopeptidase activity in this tissue was low. Two crab carboxypeptidases were purified by chromatography on DEAE-cellulose, phenyl-Sepharose, and Sephadex G-75 to homogeneity. The molecular weight values of carboxypeptidases I and II were 40,000 and 37,000, respectively. The isoelectric point value for both carboxypeptidases was 4.5. The crab carboxypeptidases were activated by NaCl, with maximal activity of carboxypeptidases I and II at 1.0 M and 0.6 M NaCl, respectively. Using 19 N-blocked dipeptides with the general structures Bz-Gly-X and Z-Gly-X, broad substrate specificity of the purified enzymes was demonstrated. Under optimal conditions the values of K m and k cat for the hydrolysis of Bz-Gly-l-Phe, Bz-Gly-l-Arg, and Bz-Gly-l-Lys by crab carboxypeptidases were determined. Inhibition data led to classification of the crab enzymes as metallopeptidases. Both carboxypeptidases were stable under neutral and mildly alkaline conditions. In addition, the presence of 1 M NaCl decreased the thermostability of the crab carboxypeptidases.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received August 13, 1999; accepted November 19, 1999.
Rights and permissions
About this article
Cite this article
Sakharov, I., Prieto, G. Purification and Some Properties of Two Carboxypeptidases from the Hepatopancreas of the Crab Paralithodes camtschatica . Mar. Biotechnol. 2, 259–266 (2000). https://doi.org/10.1007/s101269900031
Published:
Issue Date:
DOI: https://doi.org/10.1007/s101269900031