Abstract
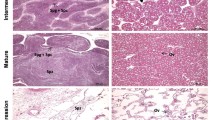
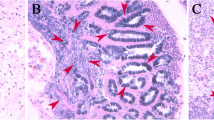
The fecundity of triploid female Crassostrea gigas exhibited significant variation and was lower compared to diploid individuals. Previous studies categorized mature stage triploid female C. gigas into two groups: female α, characterized by a high number of oocytes, and female β, displaying few or no oocytes. To investigate the molecular mechanisms underlying irregular oogenesis and fecundity differences in triploid C. gigas, we performed a comparative analysis of gonad transcriptomes at different stages of gonadal development, including female α, female β, and diploids. During early oogenesis, functional enrichment analysis between female diploids and putative female β triploids revealed differently expressed genes (DEGs) in the ribosome and ribosome biogenesis pathways. Expression levels of DEGs in these pathways were significantly decreased in the putative female β triploid, suggesting a potential role of reduced ribosome levels in obstructing triploid oogenesis. Moreover, to identify regulatory pathways in gonad development, female oysters at the early and mature stages were compared. The DNA repair and recombination proteins pathways were enriched in female diploids and female α triploids but absent in female β triploids. Overall, we propose that decreased ribosome biogenesis in female triploids hinders the differentiation of germ stem cells, leading to the formation of a large number of abnormal germ cells and ultimately resulting in reduced fecundity. The variation in fertility among triploids appeared to be related to the degree of DNA damage repair during female gonad development. This study offers valuable insights into the oogenesis process in female triploid C. gigas.







Similar content being viewed by others
Data Availability
The data analyzed in this study are presented in this article and the supplementary information file.
References
Aitken RJ (2022) Role of sperm DNA damage in creating de-novo mutations in human offspring: the ‘post-meiotic oocyte collusion’ hypothesis. Reprod Biomed Online 45:109–124
Allen SK, Downing SL (1990) Performance of triploid Pacific oysters, Crassostrea gigas: gametogenesis. Can J Fish Aquat Sci 47:1213–1222
Armistead J, Triggs-Raine B (2014) Diverse diseases from a ubiquitous process: the ribosomopathy paradox. FEBS Lett 588:1491–1500
Beaumont AR, Fairbrother JE (1991) Ploidy manipulation in molluscan shellfish: a review. J Shellfish Res 10:1–18
Breznak SM, Peng Y, Deng L, Kotb NM, Flamholz Z, Rapisarda IT, Martin ET, LaBarge KA, Fabris D, Gavis ER, Rangan P (2023) H/ACA snRNP-dependent ribosome biogenesis regulates translation of polyglutamine proteins. Sci Adv 9:eade5492
Brooks SS, Wall AL, Golzio C, Reid DW, Kondyles A, Willer JR, Botti C, Nicchitta CV, Katsanis N, Davis EE (2014) A novel ribosomopathy caused by dysfunction of RPL10 disrupts neurodevelopment and causes X-linked microcephaly in humans. Genetics 198:723–733
Cavelier P, Cau J, Morin N, Delsert C (2017) Early gametogenesis in the Pacific oyster: new insights using stem cell and mitotic markers. J Exp Biol 220:3988–3996
Chen S, Zhou Y, Chen Y, Gu J (2018) Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890
Corsini NS, Peer AM, Moeseneder P, Roiuk M, Burkard TR, Theussl HC, Moll I, Knoblich JA (2018) Coordinated control of mRNA and rRNA processing controls embryonic stem cell pluripotency and differentiation. Cell Stem Cell 22:543–558
Dégremont L, Ledu C, Maurouard E, Nourry M, Benabdelmouna A (2016) Effect of ploidy on the mortality of Crassostrea gigas spat caused by OsHV-1 in France using unselected and selected OsHV-1 resistant oysters. Aquac Res 47:777–786
Dheilly NM, Jouaux A, Boudry P, Favrel P, Lelong C (2014) Transcriptomic profiling of gametogenesis in triploid pacific oysters Crassostrea gigas: towards an understanding of partial sterility associated with triploidy. PLoS ONE 9
Forchhammer J, Lindahl L (1971) Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol 55:563–568
Galindo-Torres P, Abreu-Goodger C, Llera-Herrera R, Escobedo-Fregoso C, García-Gasca A, Ibarra AM (2022) Triploid-induced complete sterility in the scallop Nodipecten subnodosus might be triggered by an early and sustained DNA damage response. Aquaculture 559:738422
Garner E, Smogorzewska A (2011) Ubiquitylation and the fanconi anemia pathway. FEBS Lett 585:2853–2860
Guo X, Allen SK (1994) Reproductive potential and genetics of triploid Pacific oysters, Crassostrea gigas (Thunberg). Biol Bull 187:309–318
Guo X, Allen SK (1997) Sex and meiosis in autotetraploid pacific oyster, Crassostrea gigas (Thunberg). Genome 40:397–405
Higa-Nakamine S, Suzuki T, Uechi T, Chakraborty A, Nakajima Y, Nakamura M, Hirano N, Suzuki T, Kenmochi N (2012) Loss of ribosomal RNA modification causes developmental defects in zebrafish. Nucleic Acids Res 40:391–398
Jang S, Lee J, Mathews J, Ruess H, Williford AO, Rangan P, Betrán E, Buszczak M (2021) The Drosophila ribosome protein S5 paralog RpS5b promotes germ cell and follicle cell differentiation during oogenesis. Dev 148
Joo W, Xu G, Persky NS, Smogorzewska A, Rudge DG, Buzovetsky O, Elledge SJ, Pavletich NP (2011) Structure of the FANCI-FANCD2 complex: insights into the Fanconi anemia DNA repair pathway. Science. 2011;333(6040):312–316
Jouaux A, Heude-Berthelin C, Sourdaine P, Mathieu M, Kellner K (2010) Gametogenic stages in triploid oysters Crassostrea gigas: irregular locking of gonial proliferation and subsequent reproductive effort. J Exp Mar Bio Ecol 395:162–170
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y (2007) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36:D480–D484
Khajuria RK, Munschauer M, Ulirsch JC, Fiorini C, Ludwig LS, McFarland SK, Abdulhay NJ, Specht H, Keshishian H, Mani DRR et al (2018) Ribosome levels selectively regulate translation and lineage commitment in human hematopoiesis. Cell 173:90–103
Khatri P, Draghici S (2005) Ontological analysis of gene expression data: current tools, limitations, and open problems. Bioinformatics 21:3587–3595
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360
Kottemann MC, Smogorzewska A (2013) Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature 493:356–363
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079
Li Y, Xu C, Li Q (2022) Physiological and gene expression responses of diploid and triploid Pacific oyster (Crassostrea gigas) to heat acclimation. Aquac Res 53:6641–6650
Love MI, Huber W, Anders S (2014) Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:1–21
Manan H, Ikhwanuddin M (2021) Triploid induction in penaeid shrimps aquaculture: a review. Rev Aquacult 13:619–631
Martin ET, Blatt P, Nguyen E, Lahr R, Selvam S, Yoon HAM, Pocchiari T, Emtenani S, Siekhaus DE, Berman A, Fuchs G, Rangan P (2022) A translation control module coordinates germline stem cell differentiation with ribosome biogenesis during Drosophila oogenesis. Dev Cell 57:883–900
Matt JL, Allen SK (2021) A classification system for gonad development in triploid Crassostrea virginica. Aquaculture 532:735994
Mills EW, Green R (2017) Ribosomopathies: there’s strength in numbers. Science 358:6363
Murray DS, Kainz MJ, Hebberecht L, Sales KR, Hindar K, Gage MJG (2018) Comparisons of reproductive function and fatty acid fillet quality between triploid and diploid farm Atlantic salmon (Salmo salar). R Soc Open Sci 5
Nell JA (2002) Farming triploid oysters. Aquaculture 210:69–88
Normand J, Le Pennec M, Boudry P (2008) Comparative histological study of gametogenesis in diploid and triploid Pacific oysters (Crassostrea gigas) reared in an estuarine farming site in France during the 2003 heatwave. Aquaculture 282:124–129
Ogawa LM, Baserga SJ (2017) Crosstalk between the nucleolus and the DNA damage response. Mol Biosyst 13(3):443–455. 10.1039
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33:290–295
Piferrer F, Beaumont A, Falguière JC, Flajšhans M, Haffray P, Colombo L (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293:125–156
Qin JG, Fast AW, Ako H (1998) Growout performance of diploid and triploid Chinese catfish Clarias fuscus. Aquaculture 166:247–258
Sanchez CG, Teixeira FK, Czech B, Preall JB, Zamparini AL, Seifert JRK, Malone CD, Hannon GJ, Lehmann R (2016) Regulation of Ribosome Biogenesis and protein synthesis controls germline stem cell differentiation. Cell Stem Cell 18:276–290
Sondalle SB, Longerich S, Ogawa LM, Sung P, Baserga SJ (2019) Fanconi Anemia protein FANCI functions in Ribosome Biogenesis. Proc Natl Acad Sci USA 116:2561–2570
Stringer JM, Winship A, Liew SH, Hutt K (2018) The capacity of oocytes for DNA repair. Cell Mol Life Sci 75:2777–2792
Sun DF, Li Q, Yu H (2022) DNA methylation differences between male and female gonads of the oyster reveal the role of epigenetics in sex determination. Gene 820:146260
Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–12
Vitting-Seerup K, Sandelin A, Berger B (2019) IsoformSwitchAnalyzeR: analysis of changes in genome-wide patterns of alternative splicing and its functional consequences. Bioinformatics 35:4469–4471
Weber GM, Hostuttler MA, Cleveland BM, Leeds TD (2014) Growth performance comparison of intercross-triploid, induced triploid, and diploid rainbow trout. Aquaculture 433:85–93
Yang H, Simon N, Sturmer LN (2018) Production and performance of triploid oysters for aquaculture. EDIS 2018:1–9
Yang Q, Yu H, Li Q (2022a) Refinement of a classification system for gonad development in the triploid oyster Crassostrea gigas. Aquaculture 549
Yang Q, Yu H, Li Q (2022b) Disruption of cell division prevents gametogenesis in triploid Pacific oysters (Crassostrea gigas). Aquaculture 560
Yu G, Wang LG, Han Y, He QY (2012) ClusterProfiler: an R package for comparing biological themes among gene clusters. Omi A J Integr Biol 16:284–287
Zhang Q, Yu H, Howe A, Chandler W, Allen SK (2010) Cytogenetic mechanism for reversion of triploids to heteroploid mosaics in Crassostrea gigas (Thunberg) and Crassostrea ariakensis. Aquac Res 41:1658–1667
Zhang Q, Shalaby NA, Buszczak M (2014) Changes in rRNA transcription influence proliferation and cell fate within a stem cell lineage. Science 343:298–301
Acknowledgements
The authors thank Wan Sai for his experimental contributions.
Funding
This study was supported by grants from National Natural Science Foundation of China (42276111), Earmarked Fund for Agriculture Seed Improvement Project of Shandong Province (2022LZGCQY010 and 2021LZGC027), and China Agriculture Research System Project (CARS-49).
Author information
Authors and Affiliations
Contributions
Qiong Yang: Completion of the experiment, data analysis, and manuscript drafting. Hong Yu: Experimental design and coordination and manuscript revision. Qi Li: Experimental design and coordination and manuscript revision.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Yu, H. & Li, Q. Comparative Transcriptome Analysis Reveals the Role of Ribosome Reduction in Impeding Oogenesis in Female Triploid Crassostrea Gigas. Mar Biotechnol 26, 125–135 (2024). https://doi.org/10.1007/s10126-024-10283-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-024-10283-2