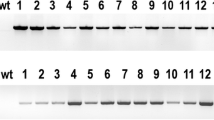
Abstract
The oleaginous diatom Fistulifera solaris is a promising producer of biofuel owing to the high content of the lipids. A genetic transformation technique by microparticle bombardment for this diatom was already established. However, the transformation efficiency was significantly lower than those of other diatoms. Devoting efforts to advance the genetic modifications of this diatom is crucial to unlock its full potential. In this study, we optimized the microparticle bombardment protocol, and newly established a multi-pulse electroporation protocol for this diatom. The nutrient-rich medium in the pre-culture stage played an essential role to increase the transformation efficiency of the bombardment method. On the other hand, use of the nutrient-rich medium in the electroporation experiments resulted in decreasing the efficiency because excess nutrient salts could hamper to establish the best conductivity condition. Adjustments on the number and voltage of the poring pulses were also critical to obtain the best balance between cell viability and efficient pore formation. Under the optimized conditions, the transformation efficiencies of microparticle bombardment and multi-pulse electroporation were 111 and 82 per 108 cells, respectively (37 and 27 times higher than the conventional bombardment method). With the aid of the optimized protocol, we successfully developed the transformant clone over-expressing the endogenous fat storage-inducing transmembrane protein (FIT)-like protein, which was previously found in the genome of the oleaginous diatom F. solaris and the oleaginous eustigmatophyte Nannochloropsis gaditana. This study provides powerful techniques to investigate and further enhance the metabolic functions of F. solaris by genetic engineering.




Similar content being viewed by others
Availability of Data and Materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Change history
02 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10126-024-10306-y
References
Apt KE, Grossman AR, Kroth-Pancic PG (1996) Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Molec Gen Genet 252:572–579
Brzezinski MA, Conley DJ (1994) Silicon deposition during the cell cycle of thalassiosira weissflogii (bacillariophyceae) determined using dual rhodamine 123 and propidium iodide staining1. J Phycol 30:45–55
Chisholm SW, Azam F, Eppley RW (1978) Silicic acid incorporation in marine diatoms on light:dark cycles: use as an assay for phased cell division 1. Limnol Oceanogr 23:518–529
Dower WJ, Miller JF, Ragsdale CW (1988) High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res 16:6127–6145
Dunahay TG, Jarvis EE, Roessler PG (1995) Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J Phycol 31:1004–1012
Faktorová D, Nisbet RER, Fernández Robledo JA et al (2020) Genetic tool development in marine protists: emerging model organisms for experimental cell biology. Nat Methods 17:481–494
Falciatore A, Casotti R, Leblanc C, Abrescia C, Bowler C (1999) Transformation of nonselectable reporter genes in marine diatoms. Mar Biotechnol 1:239–251
Ifuku K, Yan D, Miyahara M, Inoue-Kashino N, Yamamoto YY, Kashino Y (2015) A stable and efficient nuclear transformation system for the diatom Chaetoceros gracilis. Photosynth Res 123:203–211
Kadereit B, Kumar P, Wang W-J, Miranda D, Snapp EL, Severina N, Torregroza I, Evans T, Silver DL (2008) Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci U S A 105:94–99
Kashiyama Y, Ishizuka Y, Terauchi I, Matsuda T, Maeda Y, Yoshino T, Matsumoto M, Yabuki A, Bowler C, Tanaka T (2021) Engineered chlorophyll catabolism conferring predator resistance for microalgal biomass production. Metab Eng 66:79–86
Liang Y, Osada K, Sunaga Y, Yoshino T, Bowler C, Tanaka T (2015) Dynamic oil body generation in the marine oleaginous diatom Fistulifera solaris in response to nutrient limitation as revealed by morphological and lipidomic analysis. Algal Res 12:359–367
Maeda Y, Kobayashi R, Watanabe K, Yoshino T, Bowler C, Matsumoto M, Tanaka T (2022) Chromosome-scale genome assembly of the marine oleaginous diatom Fistulifera solaris. Mar Biotechnol 24:788–800
Maeda Y, Sunaga Y, Yoshino T, Tanaka T (2014) Oleosome-associated protein of the oleaginous diatom Fistulifera solaris contains an endoplasmic reticulum-targeting signal sequence. Mar Drugs 12:3892–3903
Maeda Y, Tsuru Y, Matsumoto N, Nonoyama T, Yoshino T, Matsumoto M, Tanaka T (2021a) Prostaglandin production by the microalga with heterologous expression of cyclooxygenase. Biotechnol Bioeng 118:2734–2743
Maeda Y, Watanabe K, Kaha M, Yabu Y, Yoshino T, Matsumoto M, Tanaka T (2021b) Assessment on the oil accumulation by knockdown of triacylglycerol lipase in the oleaginous diatom Fistulifera solaris. Sci Rep 11:20905
Matsumoto M, Sugiyama H, Maeda Y, Sato R, Tanaka T, Matsunaga T (2010) Marine diatom, Navicula sp. strain JPCC DA0580 and marine green alga, Chlorella sp. Strain NKG400014 as potential sources for biodiesel production. Appl Biochem Biotechnol 161:483–490
Miyagawa A, Okami T, Kira N, Yamaguchi H, Ohnishi K, Adachi M (2009) Research note: high efficiency transformation of the diatom Phaeodactylum tricornutum with a promoter from the diatom Cylindrotheca fusiformis. Phycol Res 57:142–146
Miyagawa-Yamaguchi A, Okami T, Kira N, Yamaguchi H, Ohnishi K, Adachi M (2011) Stable nuclear transformation of the diatom Chaetoceros sp.: transformation of Chaetoceros sp. Phycol Res 59:113–119
Miyahara M, Aoi M, Inoue-Kashino N, Kashino Y, Ifuku K (2013) Highly efficient transformation of the diatom Phaeodactylum tricornutum by multi-pulse electroporation. Biosci Biotechnol Biochem 77:874–876
Muto M, Fukuda Y, Nemoto M, Yoshino T, Matsunaga T, Tanaka T (2013a) Establishment of a genetic transformation system for the marine pennate diatom Fistulifera sp. strain JPCC DA0580–a high triglyceride producer. Mar Biotechnol (NY) 15:48–55
Muto M, Kubota C, Tanaka M, Satoh A, Matsumoto M, Yoshino T, Tanaka T (2013b) Identification and functional analysis of Delta-9 desaturase, a key enzyme in PUFA synthesis, isolated from the oleaginous diatom Fistulifera. PLoS ONE 8:e73507
Niu Y-F, Yang Z-K, Zhang M-H, Zhu C-C, Yang W-D, Liu J-S, Li H-Y (2012) Transformation of diatom Phaeodactylum tricornutum by electroporation and establishment of inducible selection marker. Biotechniques 52:1–3
Nojima D, Yoshino T, Maeda Y, Tanaka M, Nemoto M, Tanaka T (2013) Proteomics analysis of oil body-associated proteins in the oleaginous diatom. J Proteome Res 12:5293–5301
Nomaguchi T, Maeda Y, Liang Y, Yoshino T, Asahi T, Tanaka T (2018a) Comprehensive analysis of triacylglycerol lipases in the oleaginous diatom Fistulifera solaris JPCC DA0580 with transcriptomics under lipid degradation. J Biosci Bioeng 126:258–265
Nomaguchi T, Maeda Y, Yoshino T, Asahi T, Tirichine L, Bowler C, Tanaka T (2018b) Homoeolog expression bias in allopolyploid oleaginous marine diatom Fistulifera solaris. BMC Genomics 19:330
Osada K, Maeda Y, Yoshino T, Nojima D, Bowler C, Tanaka T (2017) Enhanced NADPH production in the pentose phosphate pathway accelerates lipid accumulation in the oleaginous diatom Fistulifera solaris. Algal Res 23:126–134
Poulsen N, Chesley PM, Kröger N (2006) Molecular genetic manipulation of the diatom thalassiosira pseudonana (bacillariophyceae). J Phycol 42:1059–1065
Poulsen N, Kröger N (2005) A new molecular tool for transgenic diatoms: control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette. FEBS J 272:3413–3423
Ryther JH, Guillard RRL (1962) Studies of marine planktonic diatoms:ii. use of cyclotella nana hustedt for assays of vitamin b12 in sea water. Can J Microbiol 8:437–445
Sakaue K, Harada H, Matsuda Y (2008) Development of gene expression system in a marine diatom using viral promoters of a wide variety of origin. Physiol Plant 133:59–67
Shin YS, Choi HI, Choi JW, Lee JS, Sung YJ, Sim SJ (2018) Multilateral approach on enhancing economic viability of lipid production from microalgae: a review. Biores Technol 258:335–344
Suhaimi N, Maeda Y, Yoshino T, Tanaka T (2022) Effects of fatty acid synthase-inhibitors on polyunsaturated fatty acid production in marine diatom Fistulifera solaris JPCC DA0580. J Biosci Bioeng 133:340–346
Sullivan CW, Volcani BE (1981) Silicon in the cellular metabolism of diatoms, In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems. Springer, New York
Sunaga Y, Maeda Y, Yabuuchi T, Muto M, Yoshino T, Tanaka T (2015) Chloroplast-targeting protein expression in the oleaginous diatom Fistulifera solaris JPCC DA0580 toward metabolic engineering. J Biosci Bioeng 119:28–34
Talebi AF, Tohidfar M, Tabatabaei M, Bagheri A, Mohsenpor M, Mohtashami SK (2013) Genetic manipulation, a feasible tool to enhance unique characteristic of Chlorella vulgaris as a feedstock for biodiesel production. Mol Biol Rep 40:4421–4428
Tanaka T, Maeda Y, Suhaimi N, Tsuneoka C, Nonoyama T, Yoshino T, Kato N, Lauersen KJ (2021) Intron-mediated enhancement of transgene expression in the oleaginous diatom Fistulifera solaris towards bisabolene production. Algal Res 57:102345
Tanaka T, Maeda Y, Veluchamy A, Tanaka M, Abida H, Maréchal E, Bowler C, Muto M, Sunaga Y, Tanaka M, Yoshino T, Taniguchi T, Fukuda Y, Nemoto M, Matsumoto M, Wong PS, Aburatani S, Fujibuchi W (2015) Oil accumulation by the oleaginous diatom Fistulifera solaris as revealed by the genome and transcriptome. Plant Cell 27:162–176
Xue G-P, Johnson JS, Dalrymple BP (1999) High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J Microbiol Methods 34:183–191
Yamashita T, Iida A, Morikawa H (1991) Evidence that more than 90% of beta-Glucuronidase-expressing cells after particle bombardment directly receive the foreign gene in their nucleus. Plant Physiol 97:829–831
Ye GN, Daniell H, Sanford JC (1990) Optimization of delivery of foreign DNA into higher-plant chloroplasts. Plant Mol Biol 15:809–819
Yi Y, Kuipers OP (2017) Development of an efficient electroporation method for rhizobacterial Bacillus mycoides strains. J Microbiol Methods 133:82–86
Yin W, Hu H (2021) High-efficiency transformation of a centric diatom Chaetoceros muelleri by electroporation with a variety of selectable markers. Algal Res 55:102274
Yuan G, Xu X, Zhang W, Zhang W, Cui Y, Qin S, Liu T (2019) Biolistic transformation of Haematococcus pluvialis with constructs based on the flanking sequences of its endogenous alpha tubulin gene. Front Microbiol 10:1749
Zaslavskaia LA, Lippmeier JC, Kroth PG, Grossman AR, Apt KE (2000) Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J Phycol 36:379–386
Zhang C, Hu H (2014) High-efficiency nuclear transformation of the diatom Phaeodactylum tricornutum by electroporation. Mar Genomics 16:63–66
Funding
This study was supported by a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO, Grant ID: JPNP17005).
Author information
Authors and Affiliations
Contributions
Insaf Naser: Formal analysis, data curation, writing original draft and editing, Yusuke Yabu: data curation, methodology, investigation, Yoshiaki Maeda: Formal analysis, writing–review and editing, Tsuyoshi Tanaka: conceptualization, review and editing, funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue - JSMB Marine Biotechnology 2022 Conference
The original online version of this article was revised: With an updated Supplementary file and Figure 3. Full information regarding the correction made can be found in the erratum/correction for this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naser, I., Yabu, Y., Maeda, Y. et al. Highly Efficient Genetic Transformation Methods for the Marine Oleaginous Diatom Fistulifera solaris. Mar Biotechnol 25, 657–665 (2023). https://doi.org/10.1007/s10126-022-10189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-022-10189-x