Abstract
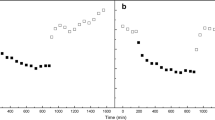
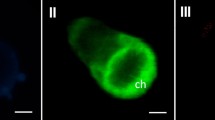
A 2,158 bp cDNA (PyBPO1) encoding a bromoperoxidase (BPO) of 625 amino acids was isolated from Pyropia yezoensis. Phylogenetic analysis using amino acid sequences of BPOs suggested that P. yezoensis and cyanobacteria were grouped in the same clade and separated from brown algae. Genomic Southern blot analysis suggested that PyBPO1 existed as a single copy per haploid genome. RT-PCR revealed that PyBPO1 was actively expressed in filamentous sporophytes but repressed in leafy gametophytes under normal growth conditions. High expression levels of PyBPO1 in sporophytes were observed when sporophytes were grown under gametophyte conditions, suggesting that preferential expression of PyBPO1 occurs during the sporophyte phase. BPO activity of cell-free extracts from sporophytes and gametophytes was examined by activity staining on native PAGE gel using o-dianisidine. One activity band was detected in sporophyte sample, but not in gametophyte sample. In addition, we found that bromide and iodide were effective substrate, but chloride was not. BPO activity was observed—likely in chloroplasts—when sporophyte cells were incubated with o-dianisidine and hydrogen peroxide. Cellular BPO staining showed the same halogen preference identified by in-gel BPO staining. Based on GS-MS analysis, bromoform was detected in medium containing sporophytes. Bromoform was not detected under dark culture conditions but was detected in the culture exposed to low light intensity (5 μmol m−2 s−1) and increased under a moderate light intensity (30 μmol m−2 s−1).







Similar content being viewed by others
References
Apt KE, Grossman AR (1993) Characterization and transcript analysis of the major phycobiliprotein subunit genes from Aglaothamnion neglectum (Rhodophyta). Plant Mol Biol 21:27–38
Asamizu E, Nakajima M, Kitade Y, Saga N, Nakamura Y, Tabata S (2003) Comparison of RNA expression profiles between the two generations of Porphyra yezoensis (Rhodophyta), based on expressed sequence tag frequency analysis. J Phycol 39:923–930
Baharum H, Chu W-C, Teo S-S, Ng K-Y, Abdul Rahim R, Ho C-L (2013) Molecular cloning, homology modeling and site-directed mutagenesis of vanadium dependent bromoperoxidase (GcVBPO1) from Gracilaria changii (Rhodophyta). Phytochemistry 92:49–59
Beissner RS, Guilford WJ, Coates RM, Hager LP (1981) Synthesis of brominated heptanones and bromoform by a bromoperoxidase of marine origin. Biochemistry 20:3727–3731
Blouin NA, Brodie JA, Grossman AC, Xu P, Brawley SH (2011) Porphyra: a marine crop shaped by stress. Trends Plant Sci 16:29–37
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram protein utilizing the principal of protein dye binding. Anal Biochem 72:248–254
Butler A, Carter-Franklin JN (2004) The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Nat Prod Rep 21:180–188
Cabrita MT, Vale C, Rauter AP (2010) Halogenated compounds from marine algae. Mar Drugs 8:2301–2317
Carter JN, Beatty KE, Simpson MT, Butler A (2002) Reactivity of recombinant and mutant vanadium bromoperoxidase from the red alga Corallina officinalis. J Inorg Biochem 91:59–69
Cock JM, Sterck L, Rouzé P, Scornet D, Allen AE, Amoutzias G, Anthouard V, Artiguenave F, Aury JM, Badger JH et al (2010) The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465:617–621
Colin C, Leblanc C, Wagner E, Delage L, Leize-Wagner E, Van Dorsselaer A, Kloareg B, Potin P (2003) The brown algal kelp Laminaria digitata features distinct bromoperoxidase and iodoperoxidase activities. J Biol Chem 278:23545–23552
Colin C, Leblanc C, Michel G, Wagner E, Leize-Wagner E, Van Dorsselaer A, Potin P (2005) Vanadium-dependent iodoperoxidases in Laminaria digitata, a novel biochemical function diverging from brown algal bromoperoxidases. J Biol Inorg Chem 10:155–166
Collén J, Porcel B, Carré W, Ball SG, Chaparro C, Tonon T et al (2013) Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc Natl Acad Sci U S A 110:5247–5252
Cowan M (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Crépineau F, Roscoe T, Kaas R, Kloareg B, Boyen C (2000) Characterisation of complementary DNAs from the expressed sequence tag analysis of life cycle stages of Laminaria digitata (Phaeophyceae). Plant Mol Biol 43:503–513
Everett RR, Kanofsky JR, Butler A (1990) Mechanistic investigations of the novel non-heme vanadium bromoperoxidases. J Biol Chem 265:4908–4914
Fukui Y, Abe M, Kobayashi M, Yano Y, Satomi M (2014) Isolation of Hyphomonas strains that induce normal morphogenesis in protoplasts of the marine red alga Pyropia yezoensis. Microb Ecol 68:556–566
Gretz MR, Aronson JM, Sommerfeld MR (1984) Taxonomic significance of cellulosic cell walls in the Bangiales (Rhodophyta). Phytochemistry 23:2513–2514
Gribble GW (2003) The diversity of naturally produced organohalogens. Chemosphere 52:289–297
Gupta V, Baghel RS, Kumar M, Kumari P, Mantri VA, Reddy CRK, Jha B (2011) Growth and agarose characteristics of isomorphic gametophyte (male and female) and sporophytes of Gracilaria dura and their marker assisted selection. Aquaculture 318:389–396
Hofsen AV, Pedersén M (1980) Bromine location in the red alga Odonthalia dentata. Z Pflanzenphysiol 96:115–122
Hofsen AV, Liljesvan B, Pedersén M (1977) Localization of bromine in the chloroplasts of the red alga Lenormandia prolifera. Bot Mar 20:267–270
Isupov MN, Dalby AR, Brindley AA, Izumi Y, Tanabe T, Murshudov GN, Littlechild JA (2000) Crystal structure of dodecameric vanadium dependent bromoperoxidase from the red algae Corallina officinalis. J Mol Biol 299:1035–1049
Johnson TL, Palenik B, Brahamsha B (2011) Characterization of a functional vanadium-dependent bromoperoxidase in the marine cyanobacteria Synechococcus sp. CC9311. J Phycol 47:792–801
Kuwano K, Aruga Y, Saga N (1996) Cryopreservation of clonal gametophytic thalli of Porphyra (Rhodophyta). Plant Sci 116:117–124
Kwon MJ, Nam TJ (2006) Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci 79:1956–1962
Latham H (2008) Temperature stress-induced bleaching of the coralline alga Corallina officinalis: a role for the enzyme bromoperoxidase. Biosci Horiz 1:104–113
Laturnus F (2001) Marine macroalgae in polar regions as natural sources for volatile organohalogens. Environ Sci Pollut Res 8:103–108
Laturnus F, Wiencke C, Kloser H (1996) Antarctic macroalgae-sources of volatile halogenated organic compounds. Mar Environ Res 41:169–181
Manley SL (2002) Phytogenesis of halomethanes: a product of selection or a metabolic accident? Biogeochemistry 60:163–180
Manley SL, Chapman DJ (1979) Metabolism of L-tyrosine to 4-hydroxybenzaldehyde and 3-bromo-4-hyrozybenzaldehyde by chloroplast-containing fractions of Odonthalia floccosa (Esp.) Falk. Plant Physiol 64:1322–1038
Marshall RA, Harper DB, McRoberts CW, Dring MJ (1999) Volatile bromocarbons produced by Falkenbergia stages of Asparagopsis spp. (Rhodophyta). Limnol Oceanogr 44:1348–1352
Matsuo Y, Imagawa H, Nishizawa M, Shizuri Y (2005) Isolation of an algal morphogenesis inducer from a marine bacterium. Science 307:1598
Mukai LS, Craigie JS, Brown RG (1981) Chemical composition and structure of the cell walls of the conchocelis and thallus phase of Porphyra tenera (Rhodophyceae). J Phycol 17:192–198
Nakamura Y, Sasaki N, Kobayashi M, Ojima N, Yasuike M, Shigenobu Y, Satomi M, Fukuma Y, Shiwaku K, Tsujimoto A, Kobayashi T, Nakayama I, Ito F, Nakajima K, Sano M, Wada T, Kuhara S, Inouye K, Gojobori T, Ikeo K (2013) The first symbiont-free genome sequence of marine red alga, Susabi-nori (Pyropia yezoensis). PLoS One 8:e57122
Nightingale PD, Malin G, Liss PS (1995) Production of chloroform and other low molecular weight halocarbons by some species of macroalgae. Limnol Oceanogr 40:680–689
Nikaido I, Asamizu E, Nakajima M, Nakamura Y, Saga N, Tabata S (2000) Generation of 10,154 expressed sequence tags from a leafy gametophyte of a marine red alga, Porphyra yezoensis. DNA Res 7:223–227
Nozaki Y (2001) An overview. In: Steele J, Thorpe S, Turekian KK (eds) Elemental distribution in the ocean in encyclopedia of ocean sciences, vol 2. Academic Press, London, pp 840–845
Ohsawa N, Ogata Y, Okada N, Itoh N (2001) Physiological function of bromoperoxidase in the red marine alga, Corallina pilulifera: production of bromoform as an allelochemical and the simultaneous elimination of hydrogen peroxide. Phytochemistry 58:683–692
Ohshiro T, Littlechild J, Garcia-Rodriguez E, Isupov MN, Lida Y, Kobayashi T, Isumi Y (2004) Modification of halogen specificity of a vanadium-dependent bromoperoxidase. Protein Sci 13:1566–1571
Paul N, de Nys R, Steinberg P (2006) Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function. Mar Ecol Prog Ser 306:87–101
Ritter A, Ubertini M, Romac S, Gaillard F, Delage L, Mann A, Cock JM, Tonon T, Correa JA, Potin P (2010) Copper stress proteomics highlights local adaptation of two strains of the model brown alga Ectocarpus siliculosus. Proteomics 10:2074–2088
Saga N (2012) Porphyra: model plants in marine sciences. In: Mikami K (ed) Porphyra yezoensis: frontiers in physiological and molecular biological research. Nova, Inc, New York, pp 1–14
Sandy M, Carter-Franklin JN, Martin JD, Butler A (2011) Vanadium bromoperoxidase from Delisea pulchra: enzyme-catalyzed formation of bromofuranone and attendant disruption of quorum sensing. Chem Commun 47:12086–12088
Shimonishi M, Kuwamoto S, Inoue H, Wever R, Ohshiro T, Izumi Y, Tanabe T (1998) Cloning and expression of the gene for a vanadium-dependent bromoperoxidase from a marine macro-alga, Corallina pilulifera. FEBS Lett 428:105–110
Shin ES, Hwang HJ, Kim IH, Nam TJ (2011) A glycoprotein from Porphyra yezoensis produces anti-inflammatory effects in liposaccharide-stimulated macrophages via the TLR4 signaling pathway. Int J Mol Med 28:809–815
Soedjak HS, Walker JV, Butler A (1995) Inhibition and inactivation of vanadium bromoperoxidase by the substrate hydrogen peroxide and further mechanistic studies. Biochemistry 34:12689–12696
Suzuki N, Takio S, Satoh T (1998) Light dependent expression in liverwort cells of chl/N and chlB identified as chloroplast genes involved in chlorophyll synthesis in the dark. J Plant Physiol 152:31–37
Theiler R, Cock JC, Hager LP, Siuda JF (1978) Halohydrocarbon synthesis by bromoperoxidase. Science 202:1094–1096
Uji T, Hirata R, Mikami K, Mizuta H, Saga N (2012) Molecular characterization and expression analysis of sodium pump genes in the marine red alga Porphyra yezoensis. Mol Biol Rep 39:7973–7980
Uji T, Mizuta H, Saga N (2013) Characterization of the sporophyte-preferential gene promoter from the red alga Porphyra yezoensis using transient gene expression. Mar Biotechnol 15:188–196
Weinberger F, Coquempot B, Forner S, Morin P, Kloareg B, Potin P (2007) Different regulation of haloperoxidation during agar oligosaccharide-activated defence mechanisms in two related red algae, Gracilaria sp. and Gracilaria chilensis. J Exp Bot 58:4365–4372
Winter JM, Moore BS (2009) Exploring the chemistry and biology of vanadium-dependent haloperoxidase. J Biol Chem 284:18577–18580
Wischang D, Radlow M, Schulz H, Vilter H, Viehweger L, Altmeyer MO, Kegler C, Herrmann J, Müller R, Gaillard F, Delage L, Leblanc C, Hartung J (2012) Molecular cloning, structure, and reactivity of the second bromoperoxidase from Ascophyllum nodosum. Bioorg Chem 44:25–34
Yamada H, Itoh N, Murakami S, Izumi Y (1985) New bromoperoxidase from coralline algae that brominates phenol compounds. Agric Biol Chem 49:2961–2967
Yoshizawa Y, Ametani A, Tsunehiro J, Nomura K, Itoh M, Fukui F, Kaminogawa S (1995) Macrophage stimulation activity of the polysaccharide fraction from a marine alga (Porphyra yezoensis): structure-function relationships and improved solubility. Biosci Biotechnol Biochem 59:1933–1937
Yotsukura N, Nagai K, Tanaka T, Kimura H, Morimoto K (2012) Temperature stress-induced changes in the proteomic profiles of Ecklonia cava (Laminariales, Phaeophyceae). J Appl Phycol 24:163–171
Acknowledgments
We would like to thank Dr. Naotsune Saga of Hokkaido University, Japan, for providing the gametophyte and sporophytes of P. yezoensis. This work was supported by Grants-in-Aid for Scientific Research on Priority Areas (to S.T.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN, to H.T.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuda, R., Ozgur, R., Higashi, Y. et al. Preferential Expression of a Bromoperoxidase in Sporophytes of a Red Alga, Pyropia yezoensis . Mar Biotechnol 17, 199–210 (2015). https://doi.org/10.1007/s10126-014-9608-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-014-9608-6