Abstract
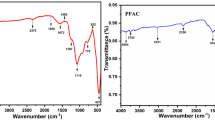
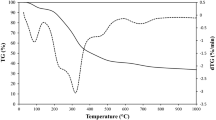
Walnut (Juglans regia) is a commonly used nutrient industrial crop but the shell of the walnut has no economic value. Hence to revamp the waste walnut shell biomass to useful product, activated carbon (AC) was prepared from J. regia shells by impregnating with NaOH. Different ACs were prepared by varying the impregnation ratio of char:NaOH as 1:1 (AC1), 1:3 (AC2), and 1:5 (AC3). The effect of impregnation ratios on the adsorptive properties of ACs for the adsorption of hexavalent chromium [Cr(VI)] was studied. The ACs were characterized by SEM, surface functionality, and zero point charge. Langmuir, Freundlich, Temkin, and Dubinin–Radushkevitch isotherm were used to interpret the batch equilibrium data. The adsorption of Cr(VI) onto ACs followed Langmuir isotherm model. Kinetic data followed pseudo second-order rate equation. Intraparticle diffusion model and Boyd plot were used to study the mechanism of the adsorption reaction. The adsorption was both by film diffusion and intraparticle diffusion. The rate-controlling step was predicted as external mass transfer. Thermodynamic parameters were also estimated. Overall, AC with higher impregnation ratio (AC3) possessed better adsorption properties compared to AC2 and AC1.





Similar content being viewed by others
References
Arranz S, Perez-Jimenez J, Saura-Calixto F (2008) Antioxidant capacity of walnut (Juglans regia L.): contribution of oil and defatted matter. Eur Food Res Technol 227:425–431
Baral SH, Das SN, Chaudhury GR, Rath P (2008) Adsorption of Cr(VI) by treated weed Salvinia cucullata: kinetics and mechanism. Adsorption 14:111–121
Biswas K, Bandhoyapadhyay D, Ghosh UC (2007) Adsorption kinetics of fluoride on iron(III)–zirconium(IV) hybrid oxide. Adsorption 13:83–94
Boehm HP (1966) Chemical identification of surface groups. Adv Catal 16:179–274
Cimino G, Passerini A, Toscano G (2000) Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res 34:2955–2962
Dewan ML, Bahadur J (2005) Uttaranchal vision and action programme. Concept Publishing Company, India
Du X, Yuan X, Li Y (2008) Equilibrium, thermodynamics and breakthrough studies for adsorption of solanesol onto macroporous resins. Chem Eng Process 47:1420–1427
El-Hendawy AA, Alexander AJ, Andrews RJ, Forest G (2008) Effect of activation schemes on porous, surface and thermal properties of activated carbons prepared from cotton stalks. J Anal Appl Pyrolysis 82:272–278
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Kumar A, Prasad B, Mishra IM (2008) Adsorptive removal of acrylonitrile by commercial grade activated carbon: kinetics, equilibrium and thermodynamics. J Hazard Mater 152:589–600
Langmuir I (1916) The adsorption of gases on plane surface of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Loannidou O, Zabaniotov A (2006) Agricultural residues as precursors for activated carbon production- a review. Renew Sustain Energy Rev 11:1966–2005
Lozano-Cartello P, Calo JM, Cazorla-Amosos D, Linares-Solano A (2007) Carbon activation with KOH as explored by temperature programmed techniques and the effect of hydrogen. Carbon 45:2529–2536
Martinez ML, Torres MM, Guzman CA, Maestri DM (2006) Preparation and characteristics of activated carbon from olive stones and walnut shells. Ind Crops Prod 23:23–28
Merrikhpour H, Jalali M (2012) Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by Iranian natural zeolite. Clean Technol Environ Policy. doi:10.1007/s10098-012-0522-1
Nahil MA, Williams PT (2012) Pore characteristics of activated carbons from the phosphoric acid chemical activation of cotton stalks. Biomass Bioenerg 57:142–147
Nowicki P, Pietragak R, Wachowska H (2008) Siberian anthracite as a precursor material for microporous activated carbons. Fuel 87:2037–2040
Panda AK, Mishra BG, Mishra DK, Singh RK (2010) Effect of sulphuric acid treatment on the physic-chemical characteristics of kaolin clay. Colloids Surf A 363:98–104
Rivera-Utrilla J, Sanchez-Polo M (2011) Adsorbent-adsorbate interactions in the adsorption of organic & inorganic species on ozonized activated carbons: a short review. Adsorption 17(3):611–620
Ubago-Perez R, Carrasco-Marin F, Fairen-Jimenez D, Moreno-Cartilla (2006) Granular and monolithic activated carbons from KOH-activation of olive stones. Microporous Mesoporous Mater 92(1):64–70
Vargas AMM, Cazetta AL, Garcia CA, Moraes JCG, Nogami EM, Lenzi E, Costa WF, Almeida VC (2011) Preparation and characterization of activated carbon from a new raw lignocellulosic material: flamboyant (Delonix regia) pods. J Environ Manag 92:178–184
Vinke P, Eijk MV, Verbree M, Voskamp AF, Bekkum HV (1994) Modification of surfaces of a gas activated carbon and a chemically activated carbon with nitric acid, hypochlorite, and ammonia. Carbon 32(4):675–686
Visa M, Pricop F, Duta A (2011) Sustainable treatment of wastewaters resulted in the textile dyeing industry. Clean Technol Environ Policy 13:855–861
Wahab OA (2007) Kinetic and isotherm studies of copper(II) removal from wastewater using various adsorbents Egypt. J Aquat Res 33(1):125–142
Yang T, Lua AC (2006) Textural and chemical properties of zinc chloride activated carbons prepared from pistachio-nut shells. Mater Chem Phys 100:438–444
Yue Z, Economy J, Bordson G (2006) Preparation and characterization of NaOH-activated carbons from phenolic resins. J Mater Chem 16:1456–1461
Acknowledgments
One of the authors S. N thanks Council for Scientific and Industrial Research (CSIR), New Delhi, India for providing Senior Research Fellowships to carry out the presented work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nethaji, S., Sivasamy, A. Removal of hexavalent chromium from aqueous solution using activated carbon prepared from walnut shell biomass through alkali impregnation processes. Clean Techn Environ Policy 16, 361–368 (2014). https://doi.org/10.1007/s10098-013-0619-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-013-0619-1