Abstract
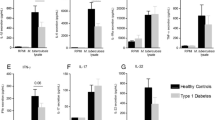
As patients with diabetes mellitus are at increased risk of developing tuberculosis, we hypothesized that this susceptibility to mycobacterial infection is due to a defective Th1-cytokine response. To explore this hypothesis, we examined four groups of subjects in Indonesia: 23 patients with tuberculosis, 34 patients with tuberculosis and diabetes, 32 patients with diabetes only and 36 healthy controls. Ex-vivo production of interferon (IFN)γ, tumour necrosis factor-α and interleukin (IL)-1β, 6, 10, -12 and -4 was measured following stimulation with Mycobacterium tuberculosis, Escherichia coli lipopolysaccharide and phytohaemagglutinin. Patients with active tuberculosis were found to have lower IFNγ levels and a higher production of other pro-inflammatory cytokines and IL-4, both in the presence and absence of diabetes. Diabetes patients without tuberculosis, however, showed strongly reduced non-specific IFNγ production, which is essential for inhibition of the initial growth of M. tuberculosis. Our data suggest that a defective non-specific immune response in diabetes may contribute to an increased susceptibility to develop tuberculosis.


Similar content being viewed by others
References
Boucot KR, Dillon ES, Cooper DA, Meier P, Richardson R (1952) Tuberculosis among diabetics: the Philadelphia survey. Am Rev Tuberc 65(1:2):1–50
Root HF (1934) The association of diabetes and tuberculosis. N Eng J Med 210:1–13
Alisjahbana B, van Crevel R, Sahiratmadja E, den Heijer M, Maya A, Istriana E et al. (2006) Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis 10(6):696–700
Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG (1995) Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis 76(6):529–533
Ponce-De-Leon A, Garcia-Garcia Md ML, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G et al. (2004) Tuberculosis and diabetes in southern Mexico. Diabetes Care 27(7):1584–1590
van Crevel R, Ottenhoff TH, van der Meer JW (2002) Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev 15(2):294–309
Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S et al (2005) Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest 115(9):2480–2488
Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD et al. (2001) Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345(15):1098–1104
Ottenhoff TH, Verreck FA, Hoeve MA, Vosse E (2005) Control of human host immunity to mycobacteria. Tuberculosis 85(1–2):53–64
Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S et al.(1999) Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis 180(6):2069–2073
Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY et al. (2002) Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol 32(6):1605–1613
Desfaits AC, Serri O, Renier G (1998) Normalization of plasma lipid peroxides, monocyte adhesion, and tumor necrosis factor-alpha production in NIDDM patients after gliclazide treatment. Diabetes Care 21(4):487–493
Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, Seljelid R (2000) Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 49(9):1451–1458
Geerlings SE, Brouwer EC, van Kessel KP, Gaastra W, Hoepelman AM (2000) Cytokine secretion is impaired in women with diabetes mellitus. Adv Exp Med Biol 485:255–262
Ohno Y, Aoki N, Nishimura A (1993) In vitro production of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 77(4):1072–1077
Sugawara I, Yamada H, Mizuno S (2004) Pulmonary tuberculosis in spontaneously diabetic goto kakizaki rats. Tohoku J Exp Med 204(2):135–145
Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K et al. (2005) Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol 139(1):57–64
Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H (2007) Tuberculosis Susceptibility of Diabetic Mice. Am J Respir Cell Mol Biol. DOI 10.1165/rcmb.2006-0478OC [Epublication ahead of print]
Word Health Organization (2002) Definition, diagnosis and classification of diabetes mellitus and its complications. Report no. WHO/NCD/NCS/99.2. Department of Noncommunicable Disease Surveillance, World Health Organization, Geneva, pp 48–49
Falk A, O’Connor JB, Pratt PC (1969) Classification of pulmonary tuberculosis. In: Diagnosis standards and classification of tuberculosis, vol 6, 12th edn. National Tuberculosis and Respiratory Disease Association, New York, pp 68–76
Sahiratmadja E, Alisjahbana B, de Boer T, Adnan I, Maya A, Danusantoso H et al. (2007) Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease. Infect Immun 75(2):820–829
Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM et al. (2005) NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 1(3):279–285
Tsukaguchi K, Okamura H, Ikuno M, Kobayashi A, Fukuoka A, Takenaka H et al. (1997) The relation between diabetes mellitus and IFN-gamma, IL-12 and IL-10 productions by CD4+ alpha beta T cells and monocytes in patients with pulmonary tuberculosis. Kekkaku 72(11):617–622
Tsukaguchi K, Okamura H, Matsuzawa K, Tamura M, Miyazaki R, Tamaki S et al.(2002) Longitudinal assessment of IFN-gamma production in patients with pulmonary tuberculosis complicated with diabetes mellitus. Kekkaku 77(5):409–413
Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA (2004) Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res 24(7):381–387
Winkler G, Dworak O, Salamon F, Salamon D, Speer G, Cseh K (1998) Increased interleukin-12 plasma concentrations in both, insulin-dependent and non-insulin-dependent diabetes mellitus. Diabetologia 41(4):488
Fischer CP, Perstrup LB, Berntsen A, Eskildsen P, Pedersen BK (2005) Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin Immunol 117(2):152–160
Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S et al. (2003) Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism 52(5):605–608
Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ et al. (2006) Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 12(6):650–656
Skeen MJ, Ziegler HK (1995) Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol 154(11):5832–5841
Netea MG, Stuyt RJ, Kim SH, Van der Meer JW, Kullberg BJ, Dinarello CA (2002) The role of endogenous interleukin (IL)-18, IL-12, IL-1beta, and tumor necrosis factor-alpha in the production of interferon-gamma induced by Candida albicans in human whole-blood cultures. J Inf Dis 185(7):963–970
Liu BF, Miyata S, Kojima H, Uriuhara A, Kusunoki H, Suzuki K et al. (1999) Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes 48(10):2074–2082
Acknowledgements
This study is an indirect result of the project “Immunogenetic basis of susceptibility to and disease manifestations of mycobacterial infectious”, conducted within the ‘Scientific Programme Indonesia Netherlands’ (SPIN) and supported by the Royal Academy of Arts and Sciences (KNAW), Netherlands, and the Poverty Related Infection Oriented Research (PRIOR) funded by NWO-WOTRO. Funding was provided by the KNAW (mobility grant), the Doctor Catharina van Tussenbroek Foundation, the Janssens Foundation, and the Nederlandse Vereniging voor Immunologie (NVVI), all from the Netherlands. We thank Frenita Siagian and Genia Soemitro for their efforts in subject management and obtaining blood samples. We also extend our gratitude to Dr. Halim Danusantoso and the staff from the Indonesian Tuberculosis Control Association, Jakarta Branch, Indonesia, and Prof. Sangkot Marzuki, director of the Eijkman Institute of Molecular Biology, Jakarta, for their kind support in this collaborative project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stalenhoef, J.E., Alisjahbana, B., Nelwan, E.J. et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis 27, 97–103 (2008). https://doi.org/10.1007/s10096-007-0395-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0395-0