Abstract
Introduction
Chronic insomnia disorder (CID) significantly impacts well-being and daily functioning. Daridorexant, a double orexin receptor blocker, has shown efficacy in randomized clinical trials and has been recently approved for the treatment of CID in adult patients. This retrospective observational study aimed to describe real-world data on daridorexant effectiveness and safety in adult patients with CID.
Methods
Consecutive patients initiating on-label daridorexant at the Sleep Medicine Centre, University Hospital of Rome Tor Vergata were enrolled. Baseline and 30-day follow-up (FU) evaluations included patients’ and CID characteristics, comorbidities, and clinicians’ and patients’ subjective ratings of changes with the Clinical and Patient Global Impression-Improvement scores (CGI-Is and PGI-Is), as well as Insomnia Severity Index (ISI) scores in a subgroup of patients.
Results
Sixty-nine patients initiated 50-mg daily dosage. At FU, 58% of both patients and clinicians rated CID as improved on CGI-Is and PGI-Is, with no differences based on comorbidities, sex, or number of previous medications. No significant predictors of CGI-Is and PGI-Is improvement were identified. At FU, ISI scores (n = 24) significantly decreased from 18.25 ± 3.21 to 12.08 ± 6.12 (Z = 8.000; p < 0.001). Of these, eight patients (33.3%) had absence of insomnia symptoms, and no patients reported a worsening in ISI score categories.
Conclusions
This study suggests daridorexant to be effective and safe in real-world CID treatment whether used as a first-ever treatment, switch, or add-on, as reflected by subjective and objective measures and the absence of serious treatment-related adverse events. Future research on larger cohorts should explore daridorexant potential across diverse patient characteristics.
Similar content being viewed by others
Introduction
Insomnia is a common condition within the general population, being the second most prevalent mental disorder and the most common sleep complaint. Chronic insomnia disorder (CID) is a prevalent sleep disorder significantly affecting individuals’ well-being and daily functioning, affecting about 10–20% of the population, especially women, older adults, and individuals of lower socioeconomic status [1]. Insomnia can be very heterogeneous in terms of clinical presentation, patients’ complaints, and underlying mechanisms and is often associated with comorbid conditions; moreover, it can precipitate the patients’ quality of life (QoL) and overall health and is a risk factor for other neurological conditions such as epilepsy, headache, and Alzheimer’s and Parkinson’s diseases [1].
It is thus crucial to treat insomnia to improve patients’ QoL and general health. One of the most recent therapeutic options takes advantage of the orexin system by blocking orexin receptors. This is of particular interest as orexins act on different biological circuits, both centrally and peripherally, involved in insomnia and other associated conditions [2]. Among these new drugs, daridorexant (a dual orexin receptor antagonists) has been recently approved for the treatment of adult patients with insomnia in North America and Europe [3,4,5,6,7,8]. Several randomized clinical trials (RCTs) showed that daridorexant significantly improved subjective and objective sleep measures, as well as daytime functioning in the short- (up to 3 months) [9,10,11,12,13] and long-term (up to 1 year) [14]. Daridorexant has good efficacy and tolerability, has limited potential for abuse and rebound effects after discontinuation, and does not negatively impact cardiac and respiratory parameters during nighttime sleep; these characteristics make it particularly useful even in cases of polypharmacy—such in the elderly—and substance misuse [2].
This study aimed to evaluate retrospective data on the effectiveness and safety of daridorexant treatment in adult patients with CID, as to provide evidence from real-world clinical practice.
Methods
The present investigation is a retrospective observational study. Consecutive patients starting on-label daridorexant 50 mg/daily per clinician’s choice at the Sleep Medicine Centre, Neurology Unit, University Hospital of Rome Tor Vergata were enrolled and evaluated at baseline and at 30-day follow-up (FU). We evaluated patients’ characteristics, CID duration, previous insomnia treatments, comorbidities, and clinicians’ and patients’ subjective ratings of changes with the Clinical and Patient Global Impression-Improvement scores (CGI-Is and PGI-Is) that are rated on 7-point Likert scale (1 = very much improved; 7 = very much worse). Changes in insomnia symptoms were evaluated with the Insomnia Severity Index (ISI) scale in a subgroup of patients.
Statistical analysis
Data analysis was. Categorical data are reported as counts and percentages. The significance group differences conducted with the statistical program SPSS for Windows version 25.0 (IBM Corp, Armonk, NY, USA). Data are reported as mean ± standard deviation in CGI-I and PCG-I outcomes were evaluated with the Mann–Whitney U test and Kruskal–Wallis test. The association of CID duration with CGI-I and PGI-I was explored through Kendall’s tau correlation analysis. Two sets of hierarchical regression analyses accounting for the respective contribution of age, CID duration, and number of previous insomnia medications were performed considering CGI-I and PGI-I as outcomes. p-values < 0.05 indicate statistical significance.
Results
Sixty-nine patients were enrolled (see Table 1 for patients’ characteristics), all starting treatment with a 50-mg daily dosage. CGI-Is and PGI-Is are available for the entire cohort. At FU, mean PGI-Is were 3.00 ± 1.25, namely, only 5 (7.2%) patients rated their CID as worsened, while it remained unchanged for 24 (34.8%) and improved for 40 (58.0%). Mean CGI-Is at FU were 2.80 ± 1.18, namely, clinicians never rated insomnia as worsened, while they rated it as unchanged for 29 (42.0%) and improved for 40 patients (58.0%). No differences in CGI-Is (χ2 = 1.591, p = 0.451) and PGI-Is (χ2 = 1.079, p = 0.583) according to the number of comorbidities and most common comorbidities were found (depression (U = 585.0, p = 0.266; U = 623.5, p = 0.107, respectively); anxiety (U = 447.50, p = 0.936; U = 443.0, p = 0.989, respectively)). Moreover, no differences in CGI-Is and PGI-Is across sexes (U = 562.50, p = 0.690; U = 579.50, p = 0.857, respectively), number of previous medications (χ2 = 5.064, p = 0.281; χ2 = 5.117, p = 0.276, respectively), and previous insomnia treatment medication use (p = 0.982; p = 0.610) were found. Further, no association between CID duration and CGI-I (τb = − 0.012, p = 0.900) and PGI-I (τb = − 0.047, p = 0.613) was found. In linear regression models, age (β = 0.030, p = 0.817; β = 0.010, p = 0.352, respectively), CID duration (β = 0.011, p = 0.930; β = − 0.076, p = 0.547, respectively), and number of previous insomnia treatment medication use (β = − 0.009, p = 0.946; β = 0.134, p = 0.303, respectively) did not predict CGI-I (F3,68 = 0.027, p = 0.994) and PGI-I (F3,68 = 0.561, p = 0.643). The model explained 3.5% of the variance for CGI-I and 15.9% of the variance for PGI-I. At FU, 12 (17.4%) dropped out, of which five (7.3%) due to inefficacy, and seven (10.2%) due to personal reasons.
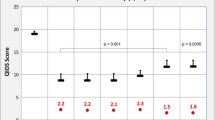
ISI evaluations were available for 24 patients. At FU, ISI scores significantly decreased from 18.25 ± 3.21 to 12.08 ± 6.12 (Z = 8.000; p < 0.001). Moreover, eight patients (33.3%) had absence of insomnia symptoms (including two with severe baseline ISI scores), seven (29.17%) reported mild symptoms, and nine (37.5%) referred symptoms of moderate severity (including one with severe baseline ISI score). Of the 18 patients with moderate ISI scores at baseline, ten (55.56%) showed improvements (four (22.22%) had no clinically significant insomnia; six (33.33%) had mild symptoms), whereas the remaining eight (44.44%) remained unchanged. Considering the three patients with mild severity ISI scores at baseline, two (66.67%) showed no clinically significant insomnia, and one (33.33%) remained in the mild category. Finally, no patients reported a worsening in ISI scores categories at FU.
Discussion
This study retrospectively evaluated the real-world daridorexant effectiveness and tolerability in unselected patients suffering from CID, mainly sleep maintenance insomnia. All patients received a 50-mg daily dosage, as this was shown to be better in improving insomnia symptoms and daytime functioning compared to lower doses [9, 10, 13]. In the subset of patients with available ISI, scores decreased significantly in 30 days. This objective change was accompanied by favorable physicians’ and patients’ perceptions of improvement as measured by CGI-Is and PGI-Is. Namely, almost 60% of both patients and clinicians judged CID as improved, while the rest rated it as unchanged, except for a small subset of five patients who rated it as worsened. Benefits were observed irrespective of whether daridorexant was used as the first-ever insomnia medication, as a switch, or as an add-on; Table 2 provides three illustrative cases of daridorexant use in these settings. No patient dropped out of the study due to treatment-related adverse events (TAE), indicating daridorexant safety.
Our results concord with the literature. Daridorexant has been evaluated in five RCTs (and post-hoc analyses), which showed its efficacy in improving several sleep outcomes such as reducing objective wake after sleep onset (WASO) and sleep latency (SL) measured after 1–2-day and 1–3-month FU, as well as improving subjective total sleep time (sTST) at 1 month [10]. Moreover, TAEs were never serious and were comparable to the placebo groups [9,10,11,12,13,14]. One long-term RCT showed daridorexant treatment for up to 12 months to be safe and well tolerated, resulting in sustained improvements in sleep and daytime functioning without concerns of late-emerging AEs [14].
Real-world studies also highlight daridorexant favorable profile in several objective scales, namely by significantly improving ISI scores (mean reduction of 7.0 ± 0.54 points) and subjective sleep parameters, such as sTST (mean increase of 54 ± 1.0 min), sSL (mean decrease of 23.9 ± 2.4 min), sWASO (mean decrease of 31.6 ± 3.2 min), and sleep efficiency (mean improvements of 10.5 ± 1.1%) [15]. Preliminary data from two prospective observational studies show that 1–3 months of daridorexant treatment result in improvements in health-related QoL [16], as well as in mood, anxiety, suicidal risk measures, and in the ability to regulate emotions and to reduce dysfunctional sleep beliefs which, in turn, may fuel hyperarousal [17]. Our cohort was also characterized by the presence of several comorbidities—especially depression, anxiety, epilepsy, and cognitive impairment—and polypharmacy. The results of the present study coupled with the other recent observations [17] are of particular interest as the enrolled patients with comorbid mood or anxiety disorder (or both) were taking antidepressants and/or mood stabilizers, and these findings may support the use of daridorexant in treatment-resistant CID in concomitance with polypharmacological treatments. The lack of differences in CGI-Is and PGI-Is across sexes, number of previous medications and comorbidities, and CID duration in our study, as well as the improvements in mood and emotions scores in the study by Palagini et al., suggest that daridorexant is effective regardless of the setting. For instance, in this study, daridorexant treatment was prescribed also in elderly patients with mild cognitive impairment, a group often characterized by sleep impairment [18], frailty, and multiple drug intake, substantiating the previous evidence suggesting a possible role of orexin neurotransmission downregulation for improving sleep in patients with cognitive impairment [19, 20] and showing the safety and efficacy of the drug with no increased risk of TAEs or residual effects the next morning, especially with the 50-mg dosage [12, 13]. Moreover, patients with comorbid epilepsy and insomnia were also evaluated in this study, and considering the proposed rational for the orexin receptors block in epilepsy [21], the beneficial effects of daridorexant may suggest new therapeutic targets for improving sleep and epilepsy in those patients [22].
Daridorexant efficacy and safety are probably due to its pharmaco-dynamic and pharmaco-kinetic properties. As a dual orexin receptor antagonist, daridorexant improves insomnia by selectively binding to both orexin receptors rather than inducing global sedation like GABA receptor agonists [8]. Furthermore, it presents no bioaccumulation and active metabolites, it is absorbed quickly, and its half-life is optimal for an insomnia medication (∼ 8 h) [8, 23, 24]. Its safety profile is characterized by the absence of serious both short- and long-term TAEs. Moreover, it is accompanied by the lack of withdrawal symptoms or rebound insomnia [9, 10, 14], in contrast to benzodiazepine and Z-drugs, which show dependency and drug-liking concerns [8].
Limitations
The present study has several limitations, especially those related to a retrospective observational design such as selection bias, limited control and accounting for confounding variables, and the inability to establish causal relations. Moreover, the relatively small number of enrolled patients might have hindered subgroup statistical comparisons and linear regressions. Finally, ISI scores were evaluated only in a small subset of patients.
Further studies on larger cohorts, both observational and RCTs, are warranted to explore the potential and limitations of daridorexant treatment according to patients’ and CID’s characteristics, comorbidities (especially psychiatric and neurological), and concomitant drug use. Polysomnographic studies addressing sleep architecture changes with daridorexant treatment are scarce and warranted and will be of use in increasing our understanding of how daridorexant actually exerts its clinical benefits. To date, it seems that dual orexin receptor antagonists improve sleep by increasing REM sleep and could thus be of particular interest in those conditions where REM sleep is particularly disrupted such as for anxiety and stress-related disorder [25].
Conclusion
Clinical and patients’ ratings of overall improvement at FU (CGI-I and PGI-I), significant reductions in the ISI scores, and lack of TAEs indicate that daridorexant is effective and safe in treating adult patients with CID in a real-world setting. Patients’ and insomnia’s features seem to not have affected treatment in our sample. Larger cohorts are warranted to further explore daridorexant potential and differences in clinical practice according to patients’ features.
Data Availability
Raw data can be provided by the corresponding author upon reasonable request.
References
Ferini-Strambi L, Auer R, Bjorvatn B, Castronovo V, Franco O, Gabutti L, Galbiati A, Hajak G, Khatami R, Kitajima T, McEvoy D, Nissen C, Perlis M, Pevernagie DA, Randerath W, Riemann D, Rizzo G, Van Someren E, Vgontzas A et al (2023) Insomnia disorder: clinical and research challenges for the 21st century. Eur J Neurol 28(7):2156–2167
Mogavero MP, Silvani A, Lanza G, DelRosso LM, Ferini-Strambi L, Ferri R (2023) Targeting orexin receptors for the treatment of insomnia: from physiological mechanisms to current clinical evidence and recommendations. Nat Sci Sleep 22(15):17–38. https://doi.org/10.2147/NSS.S201994
Idorsia Pharmaceuticals Ltd. QUVIVIQ Canada Product Monograph. Available from: https://www.idorsia.com/documents/com/label/quviviq-product-monograph.pdf. Accessed 3 Oct 2023
Idorsia Pharmaceuticals Ltd. QUVIVIQ Public Summary SwissPAR. https://www.swissmedic.ch/swissmedic/en/home/about-us/publications/public-summary-swiss-par/public-summary-swiss-par-quviviq.html. Accessed 3 Oct 2023
Idorsia Pharmaceuticals Ltd. QUVIVIQ SmPC. https://mhraproducts4853.blob.core.windows.net/docs/85c62eba3cf0011439721e4b802d79aa161eac19. Accessed 3 Oct 2023
Idorsia Pharmaceuticals Ltd. QUVIVIQ SmPC. Available from: https://www.ema.europa.eu/en/documents/product-information/quviviq-epar-product-information_en.pdf. Accessed 3 Oct 2023
Idorsia Pharmaceuticals Ltd. QUVIVIQ USPI. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214985s000lbl.pdf. Accessed 3 Oct 2023
Muehlan C, Roch C, Vaillant C, Dingemanse J (2023) The orexin story and orexin receptor antagonists for the treatment of insomnia. J Sleep Res 22:e13902. https://doi.org/10.1111/jsr.13902
Jiang F, Li H, Chen Y, Lu H, Ni J, Chen G (2023) Daridorexant for the treatment of insomnia disorder: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 102(7):e32754. https://doi.org/10.1097/MD.0000000000032754
Mignot E, Mayleben D, Fietze I, Leger D, Zammit G, Bassetti CLA, Pain S, Kinter DS, Roth T (2022) Investigators. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol 21(2):125–139. https://doi.org/10.1016/S1474-4422(21)00436-1 (Erratum in: Lancet Neurol. 2022 Jan 20; Erratum in: Lancet Neurol. 2022 Jun;21(6):e6)
Dauvilliers Y, Zammit G, Fietze I, Mayleben D, Seboek Kinter D, Pain S, Hedner J (2020) Daridorexant, a new dual orexin receptor antagonist to treat insomnia disorder. Ann Neurol 87(3):347–356. https://doi.org/10.1002/ana.25680 (Erratum in: Ann Neurol.;88(3):647–651)
Zammit G, Dauvilliers Y, Pain S, SebökKinter D, Mansour Y, Kunz D (2020) Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology 94(21):e2222–e2232. https://doi.org/10.1212/WNL.0000000000009475
Fietze I, Bassetti C, Mayleben D, Gimona A, Pain S, McCall W, Kinter DS (2022) Effects of daridorexant on sleep and daytime functioning in older adults with insomnia. Am J Geriatr Psychiatry 30(4):S69–S70
Kunz D, Dauvilliers Y, Benes H, Garcia-Borreguero D, Plazzi G, SeboekKinter D, Coloma P, Rausch M, Sassi-Sayadi M, Thein S (2023) Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs 37(1):93–106
Williams SG, Rodriguez-Cué D (2023) Use of daridorexant among patients with chronic insomnia: a retrospective observational analysis. J Clin Med 12(9):3240. https://doi.org/10.3390/jcm12093240
Winter Y, Apel D, Groppa S (2023) Influence of daridorexant on the health-related quality of life in patients with chronic insomnia. In: Abstract presented at the World Sleep Congress 2023, October 20 – 25, Rio de Janeiro, Brazil
Palagini L, Alfi G, Gurrieri R, Caruso V, Trivella M, Annuzzi E, Gambini M, Grenno G, Miniati M, Gemignani A (2023) Early experience with the new DORA daridorexant in patients with insomnia disorder: results of a real world study with a 3 months follow up period. In: Abstract presented at the World Sleep Congress 2023, October 20 – 25, Rio de Janeiro, Brazil
Liguori C, Placidi F, Izzi F, Spanetta M, Mercuri NB, Di Pucchio A (2020) Sleep dysregulation, memory impairment, and CSF biomarkers during different levels of neurocognitive functioning in Alzheimer’s disease course. Alzheimers Res Ther 12(1):5. https://doi.org/10.1186/s13195-019-0571-3 (Erratum.In:AlzheimersResTher.2020May8;12(1):53)
Liguori C, Nuccetelli M, Izzi F, Sancesario G, Romigi A, Martorana A, Amoroso C, Bernardini S, Marciani MG, Mercuri NB, Placidi F (2016) Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer’s disease. Neurobiol Aging 40:120–126. https://doi.org/10.1016/j.neurobiolaging.2016.01.007
Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani MG, Placidi F (2014) Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol 71(12):1498–1505. https://doi.org/10.1001/jamaneurol.2014.2510
Berteotti C, Calvello C, Liguori C (2023) Role of the orexin system in the bidirectional relation between sleep and epilepsy: new chances for patients with epilepsy by the antagonism to orexin receptors? Epilepsia 64(8):1991–2005. https://doi.org/10.1111/epi.17661
Carpi M, Palagini L, Fernandes M, Calvello C, Geoffroy PA, Miniati M, Pini S, Gemignani A, Mercuri NB, Liguori C (2023) Clinical usefulness of dual orexin receptor antagonism beyond insomnia: neurological and psychiatric comorbidities. Neuropharmacology 17(245):109815. https://doi.org/10.1016/j.neuropharm.2023.109815
Muehlan C, Heuberger J, Juif PE et al (2018) Accelerated development of the dual orexin receptor antagonist ACT-541468: integration of a microtracer in a first-in-human study. Clin Pharmacol Ther 104:1022–1029
Muehlan C, Brooks S, Zuiker R et al (2019) Multiple-dose clinical pharmacology of ACT-541468, a novel dual orexin receptor antagonist, following repeated-dose morning and evening administration. Eur Neuropsychopharmacol 29:847–857
Clark JW, Brian ML, Drummond SPA, Hoyer D, Jacobson LH (2020) Effects of orexin receptor antagonism on human sleep architecture: a systematic review. Sleep Med Rev 53:101332. https://doi.org/10.1016/j.smrv.2020.101332
Acknowledgements
The Authors wish to acknowledge Fabio Perversi for medical writing and editorial assistance, which have been carried out in compliance with the 2022 Good Publication Practice (GPP2022).
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement. Idorsia Pharmaceuticals
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The present investigation is part of the “DORMI 2023” study and was approved by the Ethical Committee of our center (identification code 52.23). All involved patients signed an informed consent to participate in the study.
Conflict of interest
Claudio Liguori participated in speaker or advisory board meetings and received consultantship and research funding from Idorsia; no other conflicts of interest related to this work are present. The other authors declare no conflicts of interest related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernandes, M., Placidi, F., Mercuri, N.B. et al. Daridorexant treatment for chronic insomnia: a real-world retrospective single-center study. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07326-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07326-w