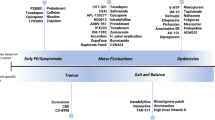
Abstract
Background
Both motor and non-motor symptoms of Parkinson’s disease (PD) have a substantial detrimental influence on the patient’s quality of life. The most effective treatment remains oral levodopa. All currently known treatments just address the symptoms; they do not completely reverse the condition.
Methodology
In order to find literature on the creation of novel treatment agents and their efficacy for PD patients, we searched PubMed, Google Scholar, and other online libraries.
Results
According to the most recent study on Parkinson’s disease (PD), a great deal of work has been done in both the clinical and laboratory domains, and some current scientists have even been successful in developing novel therapies for PD patients.
Conclusion
The quality of life for PD patients has increased as a result of recent research, and numerous innovative medications are being developed for PD therapy. In the near future, we will see positive outcomes regarding PD treatment.


Similar content being viewed by others
Data Availability
Data availability does not apply to this article as no datasets were generated or analyzed during the current study.
References
Luo Y et al (2015) Mitochondria: a therapeutic target for Parkinson’s disease? Int J Mol Sci 16(9):20704–20730
Ullah I et al (2021) Metal elements and pesticides as risk factors for Parkinson’s disease - a review. Toxicol Rep 8:607–616
Lv QK et al (2023) Role of alpha-synuclein in microglia: autophagy and phagocytosis balance neuroinflammation in Parkinson’s disease. Inflamm Res 72(3):443–462
Long T et al (2022) Ferulic acid exerts neuroprotective effects via autophagy induction in C. elegans and cellular models of Parkinson’s disease. Oxid Med Cell Longev 2022:3723567
Davidson K, Pickering AM (2023) The proteasome: a key modulator of nervous system function, brain aging, and neurodegenerative disease. Front Cell Dev Biol 11:1124907
Liu J et al (2015) Neuroprotective effects of the cultivated Chondrus crispus in a C. Elegans model of Parkinson’s disease. Marine Drugs 13(4):2250–2266
Gubert C et al (2020) Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol Dis 134
Maiti P, Manna J, Dunbar GL (2017) Current understanding of the molecular mechanisms in Parkinson's disease: targets for potential treatments. Transl Neurodegener 6(1)
Speranza L et al (2021) Dopamine: the neuromodulator of long-term synaptic plasticity, reward and movement control. Cells 10(4)
Consales C et al (2007) GDNF signaling in embryonic midbrain neurons in vitro. Brain Res 1159:28–39
Verma A et al (2020)
Gurevich EV, Gainetdinov RR, Gurevich VV (2016) G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharmacol Res 111:1–16
Chalorak P et al (2018) Holothuria scabra extracts exhibit anti-Parkinson potential in C. elegans: a model for anti-Parkinson testing. Nutr Neurosci 21(6):427–438
Kamireddy K et al (2018) Neuroprotective effect of Decalepis hamiltonii aqueous root extract and purified 2-hydroxy-4-methoxy benzaldehyde on 6-OHDA induced neurotoxicity in Caenorhabditis elegans. Biomed Pharmacother 105:997–1005
Asanuma M, Miyazaki I, Ogawa N (2003) Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res
Kim S et al (2023) Role of astrocytes in Parkinson's disease associated with genetic mutations and neurotoxicants. Cells 12(4)
Li H et al (2020) The relationship between the striatal dopaminergic neuronal and cognitive function with aging. Front Aging Neurosci 12:41
Fasano A et al (2015) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14(6):625–639
Cheng Y et al (2023) alpha-Synuclein induces prodromal symptoms of Parkinson’s disease via activating TLR2/MyD88/NF-kappaB pathway in Schwann cells of vagus nerve in a rat model. J Neuroinflammation 20(1):36
Prahl JD et al (2023) The Parkinson’s disease variant rs356182 regulates neuronal differentiation independently from alpha-synuclein. Hum Mol Genet 32(1):1–14
Smith JK, Mellick GD, Sykes AM (2023) The role of the endolysosomal pathway in α-synuclein pathogenesis in Parkinson’s disease. Front Cell Neurosci 16
Winner B et al (2011) In vivo demonstration that α-synuclein oligomers are toxic. Proc Natl Acad Sci 108(10):4194–4199
Lindström V et al (2017) Extensive uptake of α-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol Cell Neurosci 82:143–156
Burton GJ, Jauniaux E (2011) Oxidative stress. Best Pract Res Clin Obstet Gynaecol 25(3):287–299
Ge WD (2002) Free radicals in the physiological control of cell function. Physiol Rev
Obeso JA et al (2010) Missing pieces in the Parkinson’s disease puzzle. Nat Med 16(6):653–661
Trinh J, Farrer M (2013) Advances in the genetics of Parkinson disease. Nat Rev Neurol 9(8):445–454
Yao S et al (2021) A transcriptome-wide association study identifies susceptibility genes for Parkinson’s disease. NPJ Parkinsons Dis 7(1):79
Li W et al (2021) PARK genes link mitochondrial dysfunction and alpha-synuclein pathology in sporadic Parkinson’s disease. Front Cell Dev Biol 9:612476
Olufunmilayo EO, Gerke-Duncan MB, Holsinger RMD (2023) Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants (Basel) 12(2)
Sastre D et al (2023) Nuclease-dead S. aureus Cas9 downregulates alpha-synuclein and reduces mtDNA damage and oxidative stress levels in patient-derived stem cell model of Parkinson's disease. bioRxiv
Wu Y et al (2023) Vitamin B12 ameliorates the pathological phenotypes of multiple Parkinson's disease models by alleviating oxidative stress. Antioxidants (Basel) 12(1)
Korovesis D, Rubio-Tomas T, Tavernarakis N (2023) Oxidative stress in age-related neurodegenerative diseases: an overview of recent tools and findings. Antioxidants (Basel) 12(1)
Noyce AJ et al (2012) Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 72(6):893–901
Wolf SA, Boddeke HWGM, Kettenmann H (2017) Microglia in physiology and disease. Annu Rev Physiol 79(1):619–643
Grozdanov V et al (2019) Increased immune activation by pathologic α-Synuclein in parkinson’s disease. Ann Neurol 86(4):593–606
Liddelow SA et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487
Harms AS et al (2018) Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and neurodegeneration in a model of Parkinson disease. Exp Neurol 300:179–187
Matheoud D et al (2019) Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1−/− mice. Nature 571(7766):565–569
Cerri S, Blandini F (2019) Role of autophagy in Parkinson’s disease. Curr Med Chem 26(20):3702–3718
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293
Moors T et al (2016) Lysosomal dysfunction and alpha-Synuclein aggregation in Parkinson’s disease: diagnostic links. Mov Disord 31(6):791–801
Scherz-Shouval R, Elazar Z (2007) ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 17(9):422–427
Hara T et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441(7095):885–889
Webb JL et al (2003) Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278(27):25009–25013
Klucken J et al (2012) Alpha-synuclein aggregation involves a bafilomycin A 1-sensitive autophagy pathway. Autophagy 8(5):754–766
Olanow CW, McNaught KS (2006) Ubiquitin-proteasome system and Parkinson’s disease. Mov Disord 21(11):1806–1823
Mulder MPC, Witting KF, Ovaa H (2020) Cracking the ubiquitin code: the ubiquitin toolbox. Curr Issues Mol Biol 37:1–20
Thibaudeau TA, Smith DM (2019) A Practical review of proteasome pharmacology. Pharmacol Rev 71(2):170–197
Mogk A, Bukau B, Kampinga HH (2018) Cellular handling of protein aggregates by disaggregation machines. Mol Cell 69(2):214–226
Zhu Q et al (2021) Lycorine, a natural alkaloid, promotes the degradation of alpha-synuclein via PKA-mediated UPS activation in transgenic Parkinson’s disease models. Phytomedicine 87:153578
Varshavsky A (2017) The ubiquitin system, autophagy, and regulated protein degradation. Annu Rev Biochem 86:123–128
Ham SJ, Lee D, Xu WJ, Cho E, Choi S, Min S, Chung J, Park S (2021) Loss of UCHL1 rescues the defects related to Parkinson’s disease by suppressing glycolysis. Sci Adv
Webster CP et al (2017) Protein homeostasis in amyotrophic lateral sclerosis: therapeutic opportunities? Front Mol Neurosci 10:123
Wong E, Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 13(7):805–811
Smith AM et al (2018) Mitochondrial dysfunction and increased glycolysis in prodromal and early Parkinson’s blood cells. Mov Disord 33(10):1580–1590
Pickrell AM, Youle RJ (2015) The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85(2):257–273
Jiang X et al (2019) Current progress of mitochondrial quality control pathways underlying the pathogenesis of Parkinson’s disease. Oxid Med Cell Longev 2019:1–11
Goldman SM (2014) Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol 54:141–164
Hsieh C-H et al (2019) Miro1 marks Parkinson’s disease subset and Miro1 reducer rescues neuron loss in Parkinson’s models. Cell Metab 30(6):1131-1140.e7
De Deurwaerdère P, Di Giovanni G, Millan MJ (2017) Expanding the repertoire of L-DOPA’s actions: a comprehensive review of its functional neurochemistry. Prog Neurobiol 151:57–100
Tambasco N, Romoli M, Calabresi P (2018) Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol 16(8):1239–1252
LeWitt PA et al (2016) A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson’s disease. Mov Disord 31(9):1356–1365
Zhang C-L et al (2021) Research on developing drugs for Parkinson’s disease. Brain Res Bull 168:100–109
Antonini A et al (2017) Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord 45:13–20
Verhagen Metman L et al (2015) Gastroretentive carbidopa/levodopa, DM-1992, for the treatment of advanced Parkinson’s disease. Mov Disord 30(9):1222–1228
LeWitt PA et al (2013) Double-blind study of the actively transported levodopa prodrug XP21279 in Parkinson’s disease. Mov Disord 29(1):75–82
Hauser RA et al (2013) Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol 12(4):346–356
Rosebraugh M et al (2021) Foslevodopa/foscarbidopa: a new subcutaneous treatment for Parkinson’s disease. Ann Neurol 90(1):52–61
Modi NB et al (2019) Pharmacodynamics, efficacy, and safety of IPX203 in Parkinson disease patients with motor fluctuations. Clin Neuropharmacol 42(5):149–156
LeWitt PA, Giladi N, Navon N (2019) Pharmacokinetics and efficacy of a novel formulation of carbidopa-levodopa (Accordion Pill®) in Parkinson’s disease. Parkinsonism Relat Disord 65:131–138
Senek M, Nielsen EI, Nyholm D (2017) Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: a randomized crossover study. Mov Disord 32(2):283–286
Olanow CW et al (2017) A randomized trial of a low-dose Rasagiline and Pramipexole combination (P2B001) in early Parkinson’s disease. Mov Disord 32(5):783–789
Riesenberg R et al (2020) PF-06649751 efficacy and safety in early Parkinson’s disease: a randomized, placebo-controlled trial. Ther Adv Neurol Disord 13
Leta V et al (2023) The real-life effect of catechol-O-methyltransferase inhibition on non-motor symptoms in levodopa-treated Parkinson’s disease: opicapone versus entacapone. J Neural Transm 130(7):925–930
Feldman M, Margolesky J (2023) Opicapone for the treatment of Parkinson’s disease: a review. Int J Neurosci 133(5):532–543
Hattori N et al (2016) Adjunctive preladenant: a placebo-controlled, dose-finding study in Japanese patients with Parkinson’s disease. Parkinsonism Relat Disord 32:73–79
Fabrizio Stocchi M et al (2017) Randomized trial of preladenant, given as monotherapy, in patients with early Parkinson disease. Neurology
Bullock A et al (2021) Zuranolone as an oral adjunct to treatment of Parkinsonian tremor: a phase 2, open-label study. J Neurol Sci 421
Papapetropoulos S et al (2021) A phase 2 proof-of-concept, randomized, placebo-controlled trial of CX-8998 in essential tremor. Mov Disord 36(8):1944–1949
Iijima M et al (2019) Efficacy of istradefylline for gait disorders with freezing of gait in Parkinson’s disease: a single-arm, open-label, prospective, multicenter study. Expert Opin Pharmacother 20(11):1405–1411
Suzuki K et al (2018) Could istradefylline be a treatment option for postural abnormalities in mid-stage Parkinson’s disease? J Neurol Sci 385:131–133
Takahashi M et al (2022) Efficacy and safety of istradefylline in patients with Parkinson's disease presenting with postural abnormalities: results from a multicenter, prospective, and open-label exploratory study in Japan. J Neurol Sci 432
Olanow CW et al (2020) Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol 19(2):135–144
Katzenschlager R et al (2018) Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol 17(9):749–759
Katzenschlager R et al (2021) Long-term safety and efficacy of apomorphine infusion in Parkinson’s disease patients with persistent motor fluctuations: results of the open-label phase of the TOLEDO study. Parkinsonism Relat Disord 83:79–85
Hattori N et al (2020) Ropinirole patch versus placebo, ropinirole extended-release tablet in advanced Parkinson’s disease. Mov Disord 35(9):1565–1573
Hattori N et al (2020) Efficacy and safety of safinamide as an add-on therapy to L-DOPA for patients with Parkinson’s disease: a randomized, double-blind, placebo-controlled, phase II/III study. Parkinsonism Relat Disord 75:17–23
Li C et al (2020) Zonisamide for the Treatment of Parkinson disease: a current update. Front Neurosci 14
Oertel W et al (2017) Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov Disord 32(12):1701–1709
Pahwa R et al (2017) ADS-5102 (Amantadine) Extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID Study). JAMA Neurol 74(8)
Hauser RA et al (2021) Amantadine ER (Gocovri®) significantly increases ON time without any dyskinesia: pooled analyses from pivotal trials in Parkinson's disease. Front Neurol 12
Meloni M et al (2020) Efficacy and safety of 5-Hydroxytryptophan on levodopa-induced motor complications in Parkinson's disease: a preliminary finding. J Neurol Sci 415
Svenningsson P et al (2015) Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson’s disease: a dose-finding study. Brain 138(4):963–973
Perez-Lloret S, Rascol O (2018) Efficacy and safety of amantadine for the treatment of l-DOPA-induced dyskinesia. J Neural Transm 125(8):1237–1250
Wictorin K, Widner H (2016) Memantine and reduced time with dyskinesia in Parkinson′s disease. Acta Neurol Scand 133(5):355–360
Fox SH et al (2017) Trial of dextromethorphan/quinidine to treat levodopa-induced dyskinesia in Parkinson’s disease. Mov Disord 32(6):893–903
Chung KA et al (2010) Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology
Lieberman A et al (2019) Nicotine bitartrate reduces falls and freezing of gait in Parkinson disease: a reanalysis. Front Neurol 10
Moreau C et al (2012) Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. The Lancet Neurology 11(7):589–596
Markham A (2016) Pimavanserin: first global approval. Drugs 76(10):1053–1057
Mestre TA et al (2020) Glycopyrrolate improves disability from sialorrhea in parkinson’s disease: a 12-week controlled trial. Mov Disord 35(12):2319–2323
Jost WH et al (2019) SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea. Neurology 92(17):e1982–e1991
Jost WH et al (2020) Long-term incobotulinumtoxinA treatment for chronic sialorrhea: efficacy and safety over 64 weeks. Parkinsonism Relat Disord 70:23–30
Isaacson SH et al (2020) Safety and efficacy of rimabotulinumtoxinB for treatment of sialorrhea in adults: a randomized clinical trial. JAMA Neurol 77(4):461–469
Cattaneo C, Jost WH, Bonizzoni E (2020) Long-term efficacy of safinamide on symptoms severity and quality of life in fluctuating Parkinson’s disease patients. J Parkinsons Dis 10(1):89–97
Rinaldi D et al (2020) Safinamide improves executive functions in fluctuating Parkinson’s disease patients: an exploratory study. J Neural Transm 128(2):273–277
Santos García D et al (2021) Safinamide improves non-motor symptoms burden in Parkinson’s disease: an open-label prospective study. Brain Sci 11(3)
Eskow Jaunarajs KL et al (2013) Rotigotine polyoxazoline conjugate SER-214 provides robust and sustained antiparkinsonian benefit. Mov Disord 28(12):1675–1682
Müller T, Möhr J-D (2019) Pharmacokinetics of monoamine oxidase B inhibitors in Parkinson’s disease: current status. Expert Opin Drug Metab Toxicol 15(5):429–435
Mizuno Y et al (2010) Early addition of selegiline to L-dopa treatment is beneficial for patients with Parkinson disease. Clin Neuropharmacol 33(1):1–4
Chan HH et al (2018) A novel selective MAO-B inhibitor with neuroprotective and anti-Parkinsonian properties. Eur J Pharmacol 818:254–262
Zarmouh NO, Eyunni SK, Soliman KFA (2017) The Benzopyrone Biochanin-A as a reversible, competitive, and selective monoamine oxidase B inhibitor. BMC Complement Altern Med 17(1)
Blair HA, Dhillon S (2017) Safinamide: a review in Parkinson’s disease. CNS Drugs 31(2):169–176
Stocchi F et al (2012) A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early Parkinson’s disease patients. Mov Disord 27(1):106–112
Borgohain R et al (2013) Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord 29(2):229–237
Borgohain R et al (2014) Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov Disord 29(10):1273–1280
Cattaneo C et al (2017) Long-term effects of safinamide on mood fluctuations in Parkinson’s disease. J Parkinsons Dis 7(4):629–634
Peña E et al (2021) Impact of safinamide on depressive symptoms in Parkinson’s disease patients (SADness-PD Study): a multicenter retrospective study. Brain Sci 11(2)
Murata M, Hasegawa K, Kanazawa I (2007) Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology 68(1):45–50
Maeda T et al (2015) Zonisamide in the early stage of Parkinson’s disease. Neurol Clin Neurosci 3(4):127–130
Murata M et al (2015) Randomized placebo-controlled trial of zonisamide in patients with Parkinson’s disease. Neurol Clin Neurosci 4(1):10–15
Murata M et al (2015) Zonisamide improves wearing-off in Parkinson’s disease: a randomized, double-blind study. Mov Disord 30(10):1343–1350
Murata M et al (2018) Adjunct zonisamide to levodopa for DLB parkinsonism. Neurology 90(8):e664–e672
Murata M et al (2020) Effect of zonisamide on parkinsonism in patients with dementia with Lewy bodies: A phase 3 randomized clinical trial. Parkinsonism Relat Disord 76:91–97
Suzuki K et al (2021) Zonisamide effects on sleep problems and depressive symptoms in Parkinson’s disease. Brain Behav 11(3)
Morelli M, Wardas J (2001) Adenosine A2A receptor antagonists: potential therapeutic and neuroprotective effects in Parkinson's disease. Neurotox Res
Gołembiowska K, Dziubina A (2012) The Effect of adenosine A2A receptor antagonists on hydroxyl radical, dopamine, and glutamate in the striatum of rats with altered function of VMAT2. Neurotox Res 22(2):150–157
Gyoneva S et al (2014) Adenosine A2A receptor antagonism reverses inflammation-induced impairment of microglial process extension in a model of Parkinson’s disease. Neurobiol Dis 67:191–202
Yu L et al (2008) Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol 63(3):338–346
Mizuno Y, Kondo T (2013) Adenosine A2Areceptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord 28(8):1138–1141
Hazama T et al (2016) Clinical characteristics of Parkinson’s disease patients responsive to istradefylline treatment. Parkinsonism Relat Disord 22:e99–e100
Kondo T, Mizuno Y (2015) A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol 38(2):41–46
Hattori N et al (2020) A pooled analysis from phase 2b and 3 studies in Japan of istradefylline in Parkinson’s disease. Mov Disord 35(8):1481–1487
Kitta T et al (2016) Clinical efficacy of istradefylline on lower urinary tract symptoms in Parkinson’s disease. Int J Urol 23(10):893–894
Suzuki K et al (2017) Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: an open-label, 3-month study. J Neurol Sci 380:230–233
Hodgson RA et al (2010) Preladenant, a selective A2A receptor antagonist, is active in primate models of movement disorders. Exp Neurol 225(2):384–390
Hauser RA et al (2011) Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol 10(3):221–229
Hauser RA et al (2014) Tozadenant (SYN115) in patients with Parkinson’s disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised trial. Lancet Neurol 13(8):767–776
Gillespie RJ, Bamford SJ, Botting R, Comer M, Denny S, Gaur S, Griffin M, Jordan AM, Knight AR, Lerpiniere J, Leonardi S, Lightowler S, McAteer S, Merrett A, Misra A, Padfield A, Reece M, Saadi M, Selwood DL, Stratton GC, Surry D, Todd R, Tong X, Ruston V, Upton R, Weiss SM (2009) Antagonists of the human A2AAdenosine receptor. 4. Design, Synthesis, and PreclinicalEvaluation of 7-Aryltriazolo[4,5-d]pyrimidines. J Med Chem
Jamwal S, Kumar P (2019) Insight into the emerging role of striatal neurotransmitters in the pathophysiology of Parkinson’s disease and Huntington’s disease: a review. Curr Neuropharmacol 17(2):165–175
Ribeiro FM et al (2017) Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol Res 115:179–191
Reiner A, Levitz J (2018) Glutamatergic signaling in the central nervous system: ionotropic and metabotropic receptors in concert. Neuron 98(6):1080–1098
Sebastianutto I, Cenci MA (2018) mGlu receptors in the treatment of Parkinson’s disease and L-DOPA-induced dyskinesia. Curr Opin Pharmacol 38:81–89
Zhang Z et al (2019) Roles of glutamate receptors in parkinson’s disease. Int J Mol Sci 20(18)
Bezard E et al (2014) The mGluR5 negative allosteric modulator dipraglurant reduces dyskinesia in the MPTP macaque model. Mov Disord 29(8):1074–1079
Tison F et al (2016) A phase 2A trial of the novel mGluR5-Negative allosteric modulator dipraglurant for levodopa-induced dyskinesia in Parkinson’s disease. Mov Disord 31(9):1373–1380
Ikezu T et al (2010) Amantadine for dyskinesias in Parkinson's disease: a randomized controlled trial. PLoS ONE 5(12)
Goetz CG et al (2013) Which dyskinesia scale best detects treatment response? Mov Disord 28(3):341–346
Chan H-F et al (2013) Amantadine improves gait in PD patients with STN stimulation. Parkinsonism Relat Disord 19(3):316–319
Mizoguchi K, Yokoo H, Yoshida M, Tanaka T, Tanaka M (1994) Amantadine increases the extracellular dopamine levels in the striatum by re-uptake inhibition and by N-methyl-D-aspartate antagonism. Brain Res
Pahwa R et al (2015) Amantadine extended release for levodopa-induced dyskinesia in Parkinson’s disease (EASED Study). Mov Disord 30(6):788–795
Müller T, Möhr J-D (2019) Recent clinical advances in pharmacotherapy for levodopa-induced dyskinesia. Drugs 79(13):1367–1374
Malek N, Grosset DG (2014) Medication adherence in patients with Parkinson’s disease. CNS Drugs 29(1):47–53
Moreau C et al (2012) Memantine for axial signs in Parkinson’s disease: a randomised, double-blind, placebo-controlled pilot study. J Neurol Neurosurg Psychiatry 84(5):552–555
Charvin D et al (2018) An mGlu4-positive allosteric modulator alleviates parkinsonism in primates. Mov Disord 33(10):1619–1631
Berg D et al (2011) AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord 26(7):1243–1250
Stocchi F et al (2013) AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov Disord 28(13):1838–1846
Rascol O et al (2012) A proof-of-concept, randomized, placebo-controlled, multiple cross-overs (n-of-1) study of naftazone in Parkinson’s disease. Fundam Clin Pharmacol 26(4):557–564
Braak H, Del Tredici K (2017) Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis 7(s1):S71–S85
Yarnall A, Rochester L, Burn DJ (2011) The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord 26(14):2496–2503
Karachi C et al (2010) Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Investig 120(8):2745–2754
Henderson EJ et al (2016) Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15(3):249–258
Li Z et al (2015) Impact of rivastigmine on cognitive dysfunction and falling in parkinson’s disease patients. Eur Neurol 74(1–2):86–91
Dubois B et al (2012) Donepezil in Parkinson’s disease dementia: a randomized, double-blind efficacy and safety study. Mov Disord 27(10):1230–1238
Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA (2003) Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry
Quik M, Wonnacott S, Sharp BM (2011) α6β2* and α4β2* Nicotinic acetylcholine receptors as drug targets for Parkinson’s disease. Pharmacol Rev 63(4):938–966
Thacker EL, O’Reilly EJ, Weisskopf MG, Chen H, Schwarzschild M, McCullough ML, Calle EE, Thun MJ, Ascherio A (2007) Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology
Fox SH (2013) Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs 73(13):1405–1415
Villafane G et al (2007) Chronic high dose transdermal nicotine in Parkinson’s disease: an open trial. Eur J Neurol 14(12):1313–1316
Trenkwalder C et al (2016) A placebo-controlled trial of AQW051 in patients with moderate to severe levodopa-induced dyskinesia. Mov Disord 31(7):1049–1054
Di Paolo T et al (2014) AQW051, a novel and selective nicotinic acetylcholine receptor α7 partial agonist, reduces l-Dopa-induced dyskinesias and extends the duration of l-Dopa effects in parkinsonian monkeys. Parkinsonism Relat Disord 20(11):1119–1123
Arbouw MEL et al (2010) Glycopyrrolate for sialorrhea in Parkinson disease. Neurology
LeWitt PA (2012) Norepinephrine: the next therapeutics frontier for Parkinson’s disease. Transl Neurodegener
Espay AJ, LeWitt PA, Kaufmann H (2014) Norepinephrine deficiency in Parkinson’s disease: the case for noradrenergic enhancement. Mov Disord 29(14):1710–1719
Hauser RA, Hewitt LA, Isaacson S (2014) Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (NOH306A). J Parkinsons Dis 4(1):57–65
Hauser RA et al (2014) Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson’s disease (nOH306B). Mov Disord 30(5):646–654
LeWitt PA et al (2012) Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study). Neurology
Nicholson SL, Brotchie JM (2002) 5-hydroxytryptamine (5-HT, serotonin) and Parkinson's disease - opportunities for novel therapeutics to reduce the problems of levodopa therapy. Eur J Neurol
Munoz A et al (2008) Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain 131(12):3380–3394
Bezard E et al (2013) Study of the antidyskinetic effect of eltoprazine in animal models of levodopa-induced dyskinesia. Mov Disord 28(8):1088–1096
Fisher R et al (2020) The selective 5-HT1A receptor agonist, NLX-112, exerts anti-dyskinetic and anti-parkinsonian-like effects in MPTP-treated marmosets. Neuropharmacology 167
Depoortere R et al (2020) The selective 5-HT1A receptor agonist, NLX-112, exerts anti-dyskinetic effects in MPTP-treated macaques. Parkinsonism Relat Disord 78:151–157
Chang A, Fox SH (2016) Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs 76(11):1093–1118
Leucht S et al (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373(9657):31–41
Durif F, Debilly B, Galitzky M, Morand D, Viallet F, Borg M, Thobois MS, Broussolle E, Rascol O (2004) Clozapine improves dyskinesias in Parkinson disease. Neurology
Meltzer HY et al (2009) Pimavanserin, a Serotonin2A receptor inverse agonist, for the treatment of Parkinson’s disease Psychosis. Neuropsychopharmacology 35(4):881–892
Espay AJ et al (2018) Pimavanserin for Parkinson’s Disease psychosis: effects stratified by baseline cognition and use of cognitive-enhancing medications. Mov Disord 33(11):1769–1776
Chendo I, Ferreira JJ (2016) Pimavanserin for the treatment of Parkinson’s disease psychosis. Expert Opin Pharmacother 17(15):2115–2124
Prokic EJ et al (2019) Bradykinesia Is driven by cumulative beta power during continuous movement and alleviated by gabaergic modulation in Parkinson's disease. Front Neurol 10
Chen Y-Y, Sy H-N, Wu S-L (2008) Zolpidem improves akinesia, dystonia and dyskinesia in advanced Parkinson’s disease. J Clin Neurosci 15(8):955–956
Gustafsson G et al (2017) Alpha-synuclein oligomer-selective antibodies reduce intracellular accumulation and mitochondrial impairment in alpha-synuclein exposed astrocytes. J Neuroinflammation 14(1)
Tran HT et al (2014) α-Synuclein immunotherapy blocks uptake and templated propagation of misfolded α-Synuclein and neurodegeneration. Cell Rep 7(6):2054–2065
Schenk DB et al (2017) First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers. Mov Disord 32(2):211–218
Jankovic J et al (2018) Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti–α-Synuclein monoclonal antibody, in patients with Parkinson disease. JAMA Neurol 75(10)
Li Y et al (2022) Novel naturally occurring autoantibodies attenuate α‐synuclein pathology in a mouse model of Parkinson's disease. Neuropathol Appl Neurobiol 49(1)
Antonini A et al (2018) Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33(6):900–908
Shao Q-H et al (2019) Nurr1: a vital participant in the TLR4-NF-κB signal pathway stimulated by α-synuclein in BV-2 cells. Neuropharmacology 144:388–399
Decressac M et al (2013) NURR1 in Parkinson disease—from pathogenesis to therapeutic potential. Nat Rev Neurol 9(11):629–636
Smidt MP, Burbach JPH (2007) How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci 8(1):21–32
Zheng B et al (2010) PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med 2(52)
Wang J, Bi W, Zhao W, Varghese M, Koch RJ, Walker RH, Chandraratna RA, Sanders ME, Janesick A, Ward L, Blumberg B, Ho L, Pasinetti GM (2016) Selective brain penetrable Nurr1 transactivator for treating Parkinson’s disease. Oncotarget
McFarland K et al (2013) Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson’s disease. ACS Chem Neurosci 4(11):1430–1438
Kim C-H et al (2015) Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc Natl Acad Sci 112(28):8756–8761
Alcalay RN et al (2015) Glucocerebrosidase activity in Parkinson’s disease with and withoutGBAmutations. Brain 138(9):2648–2658
Du T-T et al (2015) GBA deficiency promotes SNCA/α-synuclein accumulation through autophagic inhibition by inactivated PPP2A. Autophagy 11(10):1803–1820
Atashrazm F et al (2018) Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci Rep 8(1)
Mazzulli JR et al (2011) Gaucher disease glucocerebrosidase and α-Synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146(1):37–52
McNeill A et al (2014) Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain 137(5):1481–1495
Mullin S et al (2020) Ambroxol for the treatment of patients with Parkinson disease with and without glucocerebrosidase gene mutations. JAMA Neurol 77(4)
Migdalska-Richards A et al (2016) Ambroxol effects in glucocerebrosidase and α-synuclein transgenic mice. Ann Neurol 80(5):766–775
Burbulla LF et al (2019) A modulator of wild-type glucocerebrosidase improves pathogenic phenotypes in dopaminergic neuronal models of Parkinson’s disease. Sci Transl Med 11(514)
Athauda D, Foltynie T (2016) Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog Neurobiol 145–146:98–120
Pagano G et al (2018) Diabetes mellitus and Parkinson disease. Neurology 90(19):e1654–e1662
Aviles-Olmos I et al (2013) Exenatide and the treatment of patients with Parkinson’s disease. J Clin Investig 123(6):2730–2736
Perry T, Lahiri DK, Chen D, Zhou J, Shaw KTY, Egan JM, Greig NH (2002) A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve Growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther
Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Shen H, Powers K, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Hoffer BJ, Mattsona MP, Wang Y, Greig NH (2008) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Diabetes Care
Yun SP et al (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24(7):931–938
Foltynie T (2019) Glycolysis as a therapeutic target for Parkinson’s disease. Lancet Neurol 18(12):1072–1074
Brauer R et al (2020) Diabetes medications and risk of Parkinson’s disease: a cohort study of patients with diabetes. Brain 143(10):3067–3076
Qin X et al (2021) Association between diabetes medications and the risk of Parkinson’s disease: a systematic review and meta-analysis. Front Neurol 12:678649
Wang SY et al (2020) Antidiabetic agents for treatment of Parkinson's disease: a meta-analysis. Int J Environ Res Public Health 17(13)
Jeong SH et al (2021) Beneficial effects of dipeptidyl peptidase-4 inhibitors in diabetic Parkinson’s disease. Brain 144(4):1127–1137
Holscher C (2014) First clinical data of the neuroprotective effects of nasal insulin application in patients with Alzheimer’s disease. Alzheimers Dement 10(1 Suppl):S33–S37
Novak P, Pimentel Maldonado DA, Novak V (2019) Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: a double-blinded placebo-controlled pilot study. PLoS One 14(4):e0214364
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia 60(9):1577–1585
El-Ghaiesh SH et al (2020) Metformin protects from rotenone-induced nigrostriatal neuronal death in adult mice by activating AMPK-FOXO3 signaling and mitigation of angiogenesis. Front Mol Neurosci 13:84
Ozbey G et al (2020) Metformin protects rotenone-induced dopaminergic neurodegeneration by reducing lipid peroxidation. Pharmacol Rep 72(5):1397–1406
Perez-Revuelta BI et al (2014) Metformin lowers Ser-129 phosphorylated alpha-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis 5(5):e1209
Mor DE et al (2020) Metformin rescues Parkinson’s disease phenotypes caused by hyperactive mitochondria. Proc Natl Acad Sci U S A 117(42):26438–26447
Bharath LP et al (2020) Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab 32(1):44-55 e6
Davidson MA et al (2018) Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future. Crit Rev Toxicol 48(1):52–108
Swanson C, Emborg M (2014) Expression of peroxisome proliferator-activated receptor-gamma in the substantia nigra of hemiparkinsonian nonhuman primates. Neurol Res 36(7):634–646
P P et al (2021) Glitazones activate PGC-1alpha signaling via PPAR-gamma: a promising strategy for antiparkinsonism therapeutics. ACS Chem Neurosci 12(13):2261–2272
Bonato JM et al (2018) Pioglitazone reduces mortality, prevents depressive-like behavior, and impacts hippocampal neurogenesis in the 6-OHDA model of Parkinson’s disease in rats. Exp Neurol 300:188–200
Pisanu A et al (2014) Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-gamma agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol Dis 71:280–291
He X et al (2012) Rosiglitazone protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity through inhibition of microglia activation. Int J Neurosci 122(9):532–540
Meier JJ (2012) GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 8(12):728–742
Abdelsalam RM, Safar MM (2015) Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: role of RAGE-NFkappaB and Nrf2-antioxidant signaling pathways. J Neurochem 133(5):700–707
Safar MM et al (2021) Novel mechanistic insights towards the repositioning of alogliptin in Parkinson’s disease. Life Sci 287:120132
Badawi GA et al (2019) Sitagliptin and liraglutide modulate L-dopa effect and attenuate dyskinetic movements in rotenone-lesioned rats. Neurotox Res 35(3):635–653
Funding
This work is supported by a major science and technology project of Gansu province, China (23ZDFA013-4) and Natural Science Foundation of Gansu province, China (20JR10RA602).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
No ethical approval is required for this article as no datasets were generated or analyzed during the current study.
Consent to participate
This does not apply to this article, as no datasets were generated or analyzed during the current study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ullah, I., Wang, X. & Li, H. Novel and experimental therapeutics for the management of motor and non-motor Parkinsonian symptoms. Neurol Sci (2024). https://doi.org/10.1007/s10072-023-07278-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-023-07278-7