Abstract
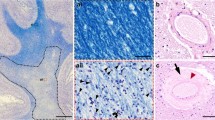
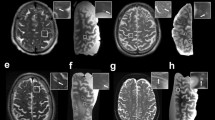
Alzheimer’s disease (AD) is the prevailing type of dementia in the elderly, yet a comprehensive comprehension of its precise underlying mechanisms remains elusive. The investigation of the involvement of cerebral small veins in the advancement of AD has yet to be sufficiently explored in previous studies, primarily due to constraints associated with pathological staining techniques. However, recent research has provided valuable insights into multiple pathophysiological occurrences concerning cerebral small veins in AD, which may manifest sequentially, concurrently, or in a self-perpetuating manner. These events are presumed to be among the initial processes in the disease’s progression. The impact of cerebral small vein loss on amyloid beta (Aβ) clearance through the glial lymphatic system is noteworthy. There exists a potential interdependence between collagen deposition and Aβ deposition in cerebral small veins. The compromised functionality of cerebral small veins can result in decreased cerebral perfusion pressure, potentially leading to cerebral tissue ischemia and edema. Additionally, the reduction of cerebral small veins may facilitate the infiltration of inflammatory factors into the brain parenchyma, thereby eliciting neuroinflammatory responses. Susceptibility-weighted imaging (SWI) is a valuable modality for the efficient assessment of cerebral small veins, precisely the deep medullary vein (DMV), and holds promise for the identification of precise and reliable imaging biomarkers for AD. This review presents a comprehensive overview of the current advancements and obstacles to the impairment of cerebral small veins in AD. Additionally, we emphasize future research avenues and the importance of conducting further investigations in this domain.

Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Morrone CD, Bishay J, McLaurin J (2020) Potential role of venular amyloid in Alzheimer’s disease pathogenesis. Int J Mol Sci 21(6):1985. https://doi.org/10.3390/ijms21061985
Marešová P, Zahálková V (2016) The economic burden of the care and treatment for people with Alzheimer’s disease: the outlook for the Czech Republic. Neurol Sci 37(12):1917–1922. https://doi.org/10.1007/s10072-016-2679-6
Tatulian SA (2022) Challenges and hopes for Alzheimer’s disease. Drug Discov Today 27(4):1027–1043. https://doi.org/10.1016/j.drudis.2022.01.016
Wang Q, Duan L, Li X, Wang Y, Guo W, Guan F et al (2022) Glucose metabolism, neural cell senescence and Alzheimer’s disease. Int J Mol Sci 23(8):4351. https://doi.org/10.3390/ijms23084351
van Harten TW, Heijmans A, van Rooden S, Wermer MJH, van Osch MJP, Kuijf HJ et al (2022) Brain deep medullary veins on 7T MRI in Dutch-type hereditary cerebral amyloid angiopathy. J Alzheimers Dis. https://doi.org/10.3233/JAD-220354
Mitra S, Gera R, Sundheimer J, Lemee M, Wahlberg LU, Linderoth B et al (2022) Microglia impairs proliferation and induces senescence in-vitro in NGF releasing cells used in encapsulated cell biodelivery for Alzheimer’s disease therapy. Int J Mol Sci 23(16):9011. https://doi.org/10.3390/ijms23169011
Low A, Prats-Sedano MA, McKiernan E, Carter SF, Stefaniak JD, Nannoni S et al (2022) Modifiable and non-modifiable risk factors of dementia on midlife cerebral small vessel disease in cognitively healthy middle-aged adults: the PREVENT-dementia study. Alzheimers Res Ther 14(1):154. https://doi.org/10.1186/s13195-022-01095-4
Liu ZY, Zhai FF, Ao DH, Han F, Li ML, Zhou L et al (2022) Deep medullary veins are associated with widespread brain structural abnormalities. J Cereb Blood Flow Metab 42(6):997–1006. https://doi.org/10.1177/0271678X211065210
Liu RM (2022) Aging, cellular senescence, and Alzheimer’s disease. Int J Mol Sci 23(4):1989. https://doi.org/10.3390/ijms23041989
Li C, Rusinek H, Chen J, Bokacheva L, Vedvyas A, Masurkar AV et al (2022) Reduced white matter venous density on MRI is associated with neurodegeneration and cognitive impairment in the elderly. Front Aging Neurosci 14:972282. https://doi.org/10.3389/fnagi.2022.972282
Xu Z, Li F, Xing D, Song H, Chen J, Duan Y et al (2021) A novel imaging biomarker for cerebral small vessel disease associated with cognitive impairment: the deep-medullary-veins score. Front Aging Neurosci 13:720481. https://doi.org/10.3389/fnagi.2021.720481
Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S et al (2021) NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc Natl Acad Sci U S A. 118(37):e2011226118. https://doi.org/10.1073/pnas.2011226118
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S et al (2019) Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22(5):719–728. https://doi.org/10.1038/s41593-019-0372-9
Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW et al (2018) beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A 115(17):4483–4488. https://doi.org/10.1073/pnas.1721694115
Keith J, Gao FQ, Noor R, Kiss A, Balasubramaniam G, Au K et al (2017) Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropathol Exp Neurol 76(4):299–312. https://doi.org/10.1093/jnen/nlx009
Bouvy WH, Kuijf HJ, Zwanenburg JJ, Koek HL, Kappelle LJ, Luijten PR et al (2017) Abnormalities of cerebral deep medullary veins on 7 Tesla MRI in amnestic mild cognitive impairment and early Alzheimer’s disease: a pilot study. J Alzheimers Dis 57(3):705–710. https://doi.org/10.3233/JAD-160952
Cortes-Canteli M, Iadecola C (2020) Alzheimer’s disease and vascular aging: JACC focus seminar. J Am Coll Cardiol 75(8):942–951. https://doi.org/10.1016/j.jacc.2019.10.062
Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC (2016) Alzheimer’s disease neuroimaging I. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 7:11934. https://doi.org/10.1038/ncomms11934
Korte N, Nortley R, Attwell D (2020) Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol 140(6):793–810. https://doi.org/10.1007/s00401-020-02215-w
Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z et al (2019) Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365(6450):eaav9518. https://doi.org/10.1126/science.aav9518
Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M et al (2019) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25(2):270–276. https://doi.org/10.1038/s41591-018-0297-y
Cruz Hernandez JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M et al (2019) Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci 22(3):413–420. https://doi.org/10.1038/s41593-018-0329-4
Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV (2016) Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta 1862(5):887–900. https://doi.org/10.1016/j.bbadis.2015.12.016
Do TM, Alata W, Dodacki A, Traversy MT, Chacun H, Pradier L et al (2014) Altered cerebral vascular volumes and solute transport at the blood-brain barriers of two transgenic mouse models of Alzheimer’s disease. Neuropharmacology 81:311–317. https://doi.org/10.1016/j.neuropharm.2014.02.010
Viticchi G, Falsetti L, Vernieri F, Altamura C, Bartolini M, Luzzi S et al (2012) Vascular predictors of cognitive decline in patients with mild cognitive impairment. Neurobiol Aging. 33(6):1127-e1. https://doi.org/10.1016/j.neurobiolaging.2011.11.027
Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC et al (2019) Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement 15(1):158–167. https://doi.org/10.1016/j.jalz.2018.07.222
Zhu W, Gao Z, Li H, Huang Z, Li X, Wang H, et al (2023) Education reduces cognitive dysfunction in Alzheimer’s disease by changing regional cerebral perfusion: an in-vivo arterial spin labeling study. Neurol Sci 44(7):2349–2361. https://doi.org/10.1007/s10072-023-06696-x
Szu JI, Obenaus A (2021) Cerebrovascular phenotypes in mouse models of Alzheimer’s disease. J Cereb Blood Flow Metab 41(8):1821–1841. https://doi.org/10.1177/0271678X21992462
Tachibana M, Yamazaki Y, Liu CC, Bu G, Kanekiyo T (2018) Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid-β pathology in amyloid model mice. Exp Neurol 300:13–21. https://doi.org/10.1016/j.expneurol.2017.10.023
Di Marco LY, Farkas E, Martin C, Venneri A, Frangi AF (2015) Is Vasomotion in cerebral arteries impaired in Alzheimer’s disease? J Alzheimers Dis 46(1):35–53. https://doi.org/10.3233/jad-142976
Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM et al (2018) Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9(1):4878. https://doi.org/10.1038/s41467-018-07318-3
Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F et al (2018) A molecular atlas of cell types and zonation in the brain vasculature. Nature 554(7693):475–480. https://doi.org/10.1038/nature25739
Liu X, Zhou Q, Zhang JH, Wang KY, Saito T, Saido TC et al (2021) Microglia-based sex-biased neuropathology in early-stage Alzheimer’s disease model mice and the potential pharmacologic efficacy of dioscin. Cells 10(11):3261. https://doi.org/10.3390/cells10113261
Brown WR, Thore CR (2011) Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 37(1):56–74. https://doi.org/10.1111/j.1365-2990.2010.01139.x
Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8(6):464–478. https://doi.org/10.1038/nrm2183
Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M (2004) Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett 366(1):80–85. https://doi.org/10.1016/j.neulet.2004.05.017
Chen X, Wei L, Wang J, Shan Y, Cai W, Men X et al (2020) Decreased visible deep medullary veins is a novel imaging marker for cerebral small vessel disease. Neurol Sci 41(6):1497–1506. https://doi.org/10.1007/s10072-019-04203-9
Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F et al (2009) Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 5(6):454–462. https://doi.org/10.1016/j.jalz.2009.04.1233
Liu Y, Dong J, Song Q, Zhang N, Wang W, Gao B et al (2020) Correlation between cerebral venous oxygen level and cognitive status in patients with Alzheimer’s disease using quantitative susceptibility mapping. Front Neurosci 14:570848. https://doi.org/10.3389/fnins.2020.570848
Ao DH, Zhang DD, Zhai FF, Zhang JT, Han F, Li ML et al (2021) Brain deep medullary veins on 3-T MRI in a population-based cohort. J Cereb Blood Flow Metab 41(3):561–568. https://doi.org/10.1177/0271678X20918467
Buée L, Hof PR, Delacourte A (1997) Brain microvascular changes in Alzheimer’s disease and other dementias. Ann N Y Acad Sci 826:7–24. https://doi.org/10.1111/j.1749-6632.1997.tb48457.x
Jorgensen DR, Shaaban CE, Wiley CA, Gianaros PJ, Mettenburg J, Rosano C (2018) A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am J Physiol Heart Circ Physiol 314(6):H1117–H1136. https://doi.org/10.1152/ajpheart.00535.2017
Shaaban CE, Aizenstein HJ, Jorgensen DR, MacCloud RL, Meckes NA, Erickson KI et al (2017) In vivo imaging of venous side cerebral small-vessel disease in older adults: an MRI method at 7T. AJNR Am J Neuroradiol 38(10):1923–1928. https://doi.org/10.3174/ajnr.A5327
Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE (2018) Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol 14(7):387–398. https://doi.org/10.1038/s41582-018-0014-y
Kalaria RN, Sepulveda-Falla D (2021) Cerebral small vessel disease in sporadic and familial Alzheimer disease. Am J Pathol 191(11):1888–1905. https://doi.org/10.1016/j.ajpath.2021.07.004
Neumann H, Wekerle H (2013) Brain microglia: watchdogs with pedigree. Nat Neurosci 16(3):253–255. https://doi.org/10.1038/nn.3338
Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15(5):300–312. https://doi.org/10.1038/nrn3722
Huang CW, Hsu SW, Chang YT, Huang SH, Huang YC, Lee CC et al (2018) Cerebral perfusion insufficiency and relationships with cognitive deficits in Alzheimer’s disease: a multiparametric neuroimaging study. Sci Rep 8(1):1541. https://doi.org/10.1038/s41598-018-19387-x
Cruz Hernández JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M et al (2019) Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci 22(3):413–420. https://doi.org/10.1038/s41593-018-0329-4
Moody DM, Brown WR, Challa VR, Anderson RL (1995) Periventricular venous collagenosis: association with leukoaraiosis. Radiology 194(2):469–476. https://doi.org/10.1148/radiology.194.2.7824728
Zhang R, Huang P, Jiaerken Y, Wang S, Hong H, Luo X et al (2021) Venous disruption affects white matter integrity through increased interstitial fluid in cerebral small vessel disease. J Cereb Blood Flow Metab 41(1):157–165. https://doi.org/10.1177/0271678X20904840
De Guio F, Vignaud A, Ropele S, Duering M, Duchesnay E, Chabriat H et al (2014) Loss of venous integrity in cerebral small vessel disease: a 7-T MRI study in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke 45(7):2124–2126. https://doi.org/10.1161/strokeaha.114.005726
Ishida K, Misawa K, Nishimura H, Hirata T, Yamamoto M, Ota N (2020) 5-Caffeoylquinic acid ameliorates cognitive decline and reduces Aβ deposition by modulating Aβ clearance pathways in APP/PS2 transgenic mice. Nutrients 12(2):494. https://doi.org/10.3390/nu12020494
Xu X, Xu H, Zhang Z (2023) Cerebral amyloid angiopathy-related cardiac injury: focus on cardiac cell death. Front Cell Dev Biol 11:1156970. https://doi.org/10.3389/fcell.2023.1156970
Jessen NA, Munk AS, Lundgaard I, Nedergaard M (2015) The glymphatic system: a beginner’s guide. Neurochem Res 40(12):2583–2599. https://doi.org/10.1007/s11064-015-1581-6
Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E et al (2016) Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 93:215–225. https://doi.org/10.1016/j.nbd.2016.05.015
Morrone CD, Liu M, Black SE, McLaurin J (2015) Interaction between therapeutic interventions for Alzheimer’s disease and physiological Aβ clearance mechanisms. Front Aging Neurosci 7:64. https://doi.org/10.3389/fnagi.2015.00064
Bugiani O (2011) Alzheimer’s disease: ageing-related or age-related? New hypotheses from an old debate. Neurol Sci 32(6):1241–1247. https://doi.org/10.1007/s10072-011-0614-4
Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ (2020) Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol 16(1):30–42. https://doi.org/10.1038/s41582-019-0281-2
Michaud JP, Bellavance MA, Prefontaine P, Rivest S (2013) Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep 5(3):646–653. https://doi.org/10.1016/j.celrep.2013.10.010
Houck AL, Gutierrez J, Gao F, Igwe KC, Colon JM, Black SE et al (2019) Increased diameters of the internal cerebral veins and the basal veins of Rosenthal are associated with white matter hyperintensity volume. AJNR Am J Neuroradiol 40(10):1712–1718. https://doi.org/10.3174/ajnr.A6213
Zlokovic BV (2010) Neurodegeneration and the neurovascular unit. Nat Med 16(12):1370–1371. https://doi.org/10.1038/nm1210-1370
Hartmann DA, Hyacinth HI, Liao FF, Shih AY (2018) Does pathology of small venules contribute to cerebral microinfarcts and dementia? J Neurochem 144(5):517–526. https://doi.org/10.1111/jnc.14228
Zhang R, Huang P, Wang S, Jiaerken Y, Hong H, Zhang Y et al (2022) Decreased cerebral blood flow and delayed arterial transit are independently associated with white matter hyperintensity. Front Aging Neurosci 14:762745. https://doi.org/10.3389/fnagi.2022.762745
Lin J, Lan L, Wang D, Qiu B, Fan Y (2017) Cerebral venous collagen remodeling in a modified white matter lesions animal model. Neuroscience 367:72–84. https://doi.org/10.1016/j.neuroscience.2017.10.031
Wang H, Huang L, Wu L, Lan J, Feng X, Li P et al (2020) The MMP-2/TIMP-2 system in Alzheimer disease. CNS Neurol Disord Drug Targets 19(6):402–416. https://doi.org/10.2174/1871527319666200812223007
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201. https://doi.org/10.1016/j.neuron.2008.01.003
Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A (2012) Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 109(4):E197-205. https://doi.org/10.1073/pnas.1111098109
Hu X, Zhou Q (2013) Health and ecosystem risks of graphene. Chem Rev 113(5):3815–3835. https://doi.org/10.1021/cr300045n
Bussi C, Peralta Ramos JM, Arroyo DS, Gallea JI, Ronchi P, Kolovou A et al (2018) Alpha-synuclein fibrils recruit TBK1 and OPTN to lysosomal damage sites and induce autophagy in microglial cells. J Cell Sci 131(23):jcs226241. https://doi.org/10.1242/jcs.226241
Griffin WST (2013) Neuroinflammatory cytokine signaling and Alzheimer’s disease. N Engl J Med 368(8):770–771. https://doi.org/10.1056/NEJMcibr1214546
Bernier M, Cunnane SC, Whittingstall K (2018) The morphology of the human cerebrovascular system. Hum Brain Mapp 39(12):4962–4975. https://doi.org/10.1002/hbm.24337
Buch S, Subramanian K, Jella PK, Chen Y, Wu Z, Shah K et al (2021) Revealing vascular abnormalities and measuring small vessel density in multiple sclerosis lesions using USPIO. Neuroimage Clin 29:102525. https://doi.org/10.1016/j.nicl.2020.102525
Taoka T, Fukusumi A, Miyasaka T, Kawai H, Nakane T, Kichikawa K et al (2017) Structure of the medullary veins of the cerebral hemisphere and related disorders. Radiographics 37(1):281–297. https://doi.org/10.1148/rg.2017160061
Raybaud C (2010) Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am 21(3):399–426. https://doi.org/10.1016/j.nec.2010.03.011
Zhang R, Zhou Y, Yan S, Zhong G, Liu C, Jiaerken Y et al (2017) A brain region-based deep medullary veins visual score on susceptibility weighted imaging. Front Aging Neurosci 9:269. https://doi.org/10.3389/fnagi.2017.00269
Kim HG, Choi JW, Han M, Lee JH, Lee HS (2020) Texture analysis of deep medullary veins on susceptibility-weighted imaging in infants: evaluating developmental and ischemic changes. Eur Radiol 30(5):2594–2603. https://doi.org/10.1007/s00330-019-06618-6
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:10. https://doi.org/10.1186/2045-8118-11-10
Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC et al (2007) Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke 38(3):888–892. https://doi.org/10.1161/01.Str.0000257998.33768.87
Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H et al (2020) Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 16(3):137–153. https://doi.org/10.1038/s41582-020-0312-z
Cao Y, Ao DH, Ma C, Qiu WY, Zhu YC (2021) Immunoreactivity and a new staining method of monocarboxylate transporter 1 located in endothelial cells of cerebral vessels of human brain in distinguishing cerebral venules from arterioles. Eur J Histochem 65(s1):3306. https://doi.org/10.4081/ejh.2021.3306.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The Clinical Research Ethics Committee of the Hospital of Chengdu University of Traditional Chinese Medicine approved this study protocol. The protocols were in accordance with the Declaration of Helsinki. Written informed consents were acquired from all participants or their legal guardians.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Z., Li, Z., Shi, Y. et al. Advancements in investigating the role of cerebral small vein loss in Alzheimer’s disease–related pathological changes. Neurol Sci 45, 1875–1883 (2024). https://doi.org/10.1007/s10072-023-07208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07208-7