Abstract
Background
The mobile device diffusion has increasingly highlighted the opportunity to collect patient-reported outcomes (PROs) through electronic patient-reported outcomes measurements (ePROMs) during the clinical routine. Despite the ePROMs promises and advantages, the equivalence when a PRO measure is moved from the original paper-and-pencil to the electronic version is still little investigated. This study aims at evaluating equivalence between PROMs and ePROMs self-administration in people with multiple sclerosis (PwMS); in addition, preference of self-administration type was evaluated.
Methods
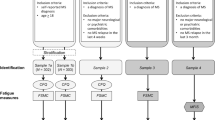
The Manual Ability Measure-36 (MAM-36) and Fatigue Severity Scale (FSS) were selected for the equivalence test. The app ABOUTCOME was developed through a user-centered design approach to administer the questionnaires on tablet. Both paper-and-pencil and electronic versions were randomly self-administered. Intrarater reliability between both versions was evaluated through the intraclass correlation coefficient (ICC, excellent for values ≥ 0.75).
Results
Fifty PwMS (35 females) participated to the study (mean age: 54.7±11.0 years, disease course: 27 relapsing-remitting and 23 progressive; mean EDSS: 4.7±1.9; mean disease duration: 13.3±9.5 years). No statistically significant differences were found for the means total scores of MAM-36 (p = 0.61) and FSS (p = 0.78). The ICC value for MAM-36 and FSS was excellent (0.98 and 0.94, respectively). Most of participants preferred the tablet version (84%).
Conclusion
The results of the study provide evidence about the equivalence between the paper-and-pencil and electronic versions of PROs administration. In addition, PwMS prefer electronic methods rather than paper because the information can be provided more efficiently and accurately. The results could be easily extended to other MS PROs.
Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Schick-Makaroff K, Molzahn A (2015) Strategies to use tablet computers for collection of electronic patient-reported outcomes. Health Qual Life Outcomes 13:2. https://doi.org/10.1186/s12955-014-0205-1
Klose K, Kreimeier S, Tangermann U, Aumann I, Damm K (2016) Patient- and person-reports on healthcare: preferences, outcomes, experiences, and satisfaction – an essay. Heal Econ Rev 6:18. https://doi.org/10.1186/s13561-016-0094-6
Griggs CL, Schneider JC, Kazis LE, Ryan CM (2017) Patient-reported outcome measures. Ann Surg 265:1066–1067. https://doi.org/10.1097/SLA.0000000000002165
Wiklund I (2004) Assessment of patient-reported outcomes in clinical trials: the example of health-related quality of life. Fundam Clin Pharmacol 18:351–363. https://doi.org/10.1111/j.1472-8206.2004.00234.x
Black N (2013) Patient reported outcome measures could help transform healthcare. BMJ 346:f167–f167. https://doi.org/10.1136/bmj.f167
Jette DU, Halbert J, Iverson C, Miceli E, Shah P (2009) Use of standardized outcome measures in physical therapist practice: perceptions and applications. Phys Ther 89:125–135. https://doi.org/10.2522/ptj.20080234
Hatfield DR, Ogles BM (2007) Why some clinicians use outcome measures and others do not. Adm Policy Ment Health Ment Health Serv Res 34:283–291. https://doi.org/10.1007/s10488-006-0110-y
Zaratin P, Vermersch P, Amato MP, Brichetto G, Coetzee T, Cutter G, Edan G, Giovannoni G, Gray E, Hartung HP, Hobart J, Helme A, Hyde R, Khan U, Leocani L, Mantovani LG, McBurney R, Montalban X, Penner I-K et al (2022) The agenda of the global patient reported outcomes for multiple sclerosis (PROMS) initiative: progresses and open questions. Mult Scler Relat Disord 61:103757. https://doi.org/10.1016/j.msard.2022.103757
Dillon DG, Pirie F, Rice S, Pomilla C, Sandhu MS, Motala AA, Young EH (2014) Open-source electronic data capture system offered increased accuracy and cost-effectiveness compared with paper methods in Africa. J Clin Epidemiol 67:1358–1363. https://doi.org/10.1016/j.jclinepi.2014.06.012
Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP (2001) Electronic diaries for monitoring chronic pain: 1-year validation study. Pain 91:277–285. https://doi.org/10.1016/S0304-3959(00)00450-4
Aiyegbusi OL (2020) Key methodological considerations for usability testing of electronic patient-reported outcome (ePRO) systems. Qual Life Res 29:325–333. https://doi.org/10.1007/s11136-019-02329-z
Burdette SD, Herchline TE, Oehler R (2008) Surfing the Web: practicing medicine in a technological age: using smartphones in clinical practice. Clin Infect Dis 47:117–122. https://doi.org/10.1086/588788
Zbrozek A, Hebert J, Gogates G, Thorell R, Dell C, Molsen E, Craig G, Grice K, Kern S, Hines S (2013) Validation of electronic systems to collect patient-reported outcome (PRO) data—recommendations for clinical trial teams: report of the ISPOR ePRO Systems Validation Good Research Practices Task Force. Value Health 16:480–489. https://doi.org/10.1016/j.jval.2013.04.002
Powell AC, Landman AB, Bates DW (2014) In search of a few good apps. JAMA 311:1851. https://doi.org/10.1001/jama.2014.2564
Brichetto G, Pedullà L, Podda J, Tacchino A (2019) Beyond center-based testing: Understanding and improving functioning with wearable technology in MS. Mult Scler:25. https://doi.org/10.1177/1352458519857075
Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH (2007) Response rate and completeness of questionnaires: a randomized study of Internet versus paper-and-pencil versions. J Med Internet Res 9:e25. https://doi.org/10.2196/jmir.9.3.e25
Gwaltney CJ, Shields AL, Shiffman S (2008) Equivalence of electronic and paper-and-pencil administration of patient-reported outcome measures: a meta-analytic review. Value Health 11:322–333. https://doi.org/10.1111/j.1524-4733.2007.00231.x
Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, Lenderking WR, Cella D, Basch E (2009) Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force Report. Value Health 12:419–429. https://doi.org/10.1111/j.1524-4733.2008.00470.x
Kimura T (2017) The impacts of computer adaptive testing from a variety of perspectives. J Educ Eval Health Prof 14:12. https://doi.org/10.3352/jeehp.2017.14.12
Rose M, Bjorner JB, Fischer F, Anatchkova M, Gandek B, Klapp BF, Ware JE (2012) Computerized adaptive testing—ready for ambulatory monitoring? Psychosom Med 74:338–348. https://doi.org/10.1097/PSY.0b013e3182547392
Cox CE, Wysham NG, Kamal AH, Jones DM, Cass B, Tobin M, White DB, Kahn JM, Hough CL, Carson SS (2016) Usability testing of an electronic patient-reported outcome system for survivors of critical illness. Am J Crit Care 25:340–349. https://doi.org/10.4037/ajcc2016952
Steele Gray C, Gill A, Khan AI, Hans PK, Kuluski K, Cott C (2016) The electronic patient reported outcome tool: testing usability and feasibility of a mobile app and portal to support care for patients with complex chronic disease and disability in primary care settings. JMIR Mhealth Uhealth 4:e58. https://doi.org/10.2196/mhealth.5331
Shahraz S, Pham TP, Gibson M, De La Cruz M, Baara M, Karnik S, Dell C, Pease S, Nigam S, Cappelleri JC, Lipset C, Zornow P, Lee J, Byrom B (2021) Does scrolling affect measurement equivalence of electronic patient-reported outcome measures (ePROM)? Results of a quantitative equivalence study. J Patient Rep Outcomes 5:23. https://doi.org/10.1186/s41687-021-00296-z
D’Amico E, Haase R, Ziemssen T (2019) Review: Patient-reported outcomes in multiple sclerosis care. Mult Scler Relat Disord 33:61–66. https://doi.org/10.1016/j.msard.2019.05.019
Walter SD, Eliasziw M, Donner A (1998) Sample size and optimal designs for reliability studies. Stat Med 17:101–110. https://doi.org/10.1002/(SICI)1097-0258(19980115)17:1<101::AID-SIM727>3.0.CO;2-E
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Solaro C, Di Giovanni R, Grange E, Brichetto G, Mueller M, Tacchino A, Bertoni R, Patti F, Pappalardo A, Prosperini L, Castelli L, Rosato R, Cattaneo D, Marengo D (2020) Italian translation and psychometric validation of the Manual Ability Measure-36 (MAM-36) and its correlation with an objective measure of upper limb function in patients with multiple sclerosis. Neurol Sci 41. https://doi.org/10.1007/s10072-020-04263-2
Ottonello M, Pellicciari L, Giordano A, Foti C (2016) Rasch analysis of the Fatigue Severity Scale in Italian subjects with multiple sclerosis. J Rehabil Med 48:597–603. https://doi.org/10.2340/16501977-2116
Allen-Philbey K, Middleton R, Tuite-Dalton K, Baker E, Stennett A, Albor C, Schmierer K (2020) Can we improve the monitoring of people with multiple sclerosis using simple tools, data sharing, and patient engagement? Front Neurol 11. https://doi.org/10.3389/fneur.2020.00464
Inojosa H, Proschmann U, Akgün K, Ziemssen T (2021) Should we use clinical tools to identify disease progression? Front Neurol 11. https://doi.org/10.3389/fneur.2020.628542
Manouchehrinia A, Zhu F, Piani-Meier D, Lange M, Silva DG, Carruthers R, Glaser A, Kingwell E, Tremlett H, Hillert J (2019) Predicting risk of secondary progression in multiple sclerosis: a nomogram. Mult Scler J 25:1102–1112. https://doi.org/10.1177/1352458518783667
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L et al (2014) Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 83:278–286. https://doi.org/10.1212/WNL.0000000000000560
van der Walt A, Butzkueven H, Shin RK, Midaglia L, Capezzuto L, Lindemann M, Davies G, Butler LM, Costantino C, Montalban X (2021) Developing a digital solution for remote assessment in multiple sclerosis: from concept to software as a medical device. Brain Sci 11:1247. https://doi.org/10.3390/brainsci11091247
Lejbkowicz I, Caspi O, Miller A (2012) Participatory medicine and patient empowerment towards personalized healthcare in multiple sclerosis. Expert Rev Neurother 12:343–352. https://doi.org/10.1586/ern.11.161
Brichetto G, Monti Bragadin M, Fiorini S, Battaglia MA, Konrad G, Ponzio M, Pedullà L, Verri A, Barla A, Tacchino A (2020) The hidden information in patient-reported outcomes and clinician-assessed outcomes: multiple sclerosis as a proof of concept of a machine learning approach. Neurol Sci 41:459–462. https://doi.org/10.1007/s10072-019-04093-x
Sun S, Folarin AA, Ranjan Y, Rashid Z, Conde P, Stewart C, Cummins N, Matcham F, Dalla Costa G, Simblett S, Leocani L, Lamers F, Sørensen PS, Buron M, Zabalza A, Guerrero Pérez AI, Penninx BW, Siddi S, Haro JM et al (2020) Using smartphones and wearable devices to monitor behavioral changes during COVID-19. J Med Internet Res 22:e19992. https://doi.org/10.2196/19992
Bush NE, Skopp N, Smolenski D, Crumpton R, Fairall J (2013) Behavioral screening measures delivered with a smartphone app. J Nerv Ment Dis 201:991–995. https://doi.org/10.1097/NMD.0000000000000039
Garcia-Palacios A, Herrero R, Belmonte MA, Castilla D, Guixeres J, Molinari G, Baños RM, Botella C (2014) Ecological momentary assessment for chronic pain in fibromyalgia using a smartphone: A randomized crossover study. Eur J Pain 18:862–872. https://doi.org/10.1002/j.1532-2149.2013.00425.x
Bierbrier R, Lo V, Wu RC (2014) Evaluation of the accuracy of smartphone medical calculation apps. J Med Internet Res 16:e32. https://doi.org/10.2196/jmir.3062
Teixeira Neto NC, Lima YL, Almeida GPL, Bezerra MA, Lima PODP, de Oliveira RR (2018) Physiotherapy questionnaires app to deliver main musculoskeletal assessment questionnaires: development and validation study. JMIR Rehabil Assist Technol 5:e1. https://doi.org/10.2196/rehab.9247
Marcano Belisario JS, Jamsek J, Huckvale K, O’Donoghue J, Morrison CP, Car J (2015) Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.MR000042.pub2
Feys P, Giovannoni G, Dijsselbloem N, Centonze D, Eelen P, Lykke Andersen S (2016) The importance of a multi-disciplinary perspective and patient activation programmes in MS management. Mult Scler J 22:34–46. https://doi.org/10.1177/1352458516650741
Marziniak M, Brichetto G, Feys P, Meyding-Lamadé U, Vernon K, Meuth SG (2018) The use of digital and remote communication technologies as a tool for multiple sclerosis management: narrative review. JMIR Rehabil Assist Technol 5:e5. https://doi.org/10.2196/rehab.7805
Cancela J, Charlafti I, Colloud S, Wu C (2021) Digital health in the era of personalized healthcare. In: Digital Health. Elsevier, pp 7–31
Brichetto G (2020) We should monitor our patients with wearable technology instead of neurological examination – commentary. Mult Scler J 26:1028–1030. https://doi.org/10.1177/1352458520930985
Salimzadeh Z, Damanabi S, Kalankesh L, Ferdousi R (2019) Mobile applications for multiple sclerosis: a focus on self-management. Acta Inform Med 27:12. https://doi.org/10.5455/aim.2019.27.12-18
Acknowledgements
We would like to acknowledge the significant contribution of Maria Madera and Giulia Bignone (secretariat) to the implementation of this protocol.
Funding
This work was supported by the Italian Multiple Sclerosis Foundation (FISM) (grant number 2014/20).
Author information
Authors and Affiliations
Contributions
All the authors approved the submitted version (and any substantially modified version that involves the author’s contribution to the study) and agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. In addition, each of the authors made specific contributions as outlined below. AT substantially contributed to the conception and design of the work; and to the acquisition, analysis, and interpretation of data; he drafted the work. RDG substantially contributed to the conception of the work; and to the acquisition, and analysis of data; she revised the work critically for important intellectual content. EG substantially contributed to the conception of the work; and to the acquisition, and analysis of data; she revised the work critically for important intellectual content. MMS substantially contributed to the conception of the work; she revised the work critically for important intellectual content. MP substantially contributed to the analysis and interpretation of data; she revised the work critically for important intellectual content. MAB substantially contributed to the conception and design of the work. He revised the work critically for important intellectual content. GB substantially contributed to the conception and design of the work; he revised the work critically for important intellectual content. CS substantially contributed to the conception and design of the work; and to the acquisition, analysis, and interpretation of data; he revised the work critically for important intellectual content.
Corresponding author
Ethics declarations
Ethics approval
The project was approved by the local Ethics Committee of San Martino Hospital (P.R.196REG2015) and performed in accordance with the ethical standards as laid down in the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tacchino, ., Di Giovanni, R., Grange, E. et al. The administration of the paper and electronic versions of the Manual Ability Measure-36 (MAM-36) and Fatigue Severity Scale (FSS) is equivalent in people with multiple sclerosis. Neurol Sci 45, 1155–1162 (2024). https://doi.org/10.1007/s10072-023-07103-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07103-1