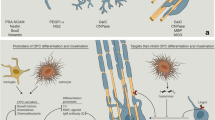
Abstract
Multiple sclerosis (MS) is a chronic autoimmune and demyelinating disease of the central nervous system (CNS) which leads to focal demyelinated lesions in the brain and spinal cord. Failure of remyelination contributes to chronic disability in young adults. Characterization of events occurring during the demyelination and remyelination processes and those of which subsequently limit remyelination or contribute to demyelination can provide the possibility of new therapies development for MS. Most of the currently available therapies and investigations modulate immune responses and mediators. Since most therapeutic strategies have unsatisfied outcomes, developing new therapies that enhance brain lesion repair is a priority. A close look at cellular and chemical components of MS lesions will pave the way to a better understanding of lesions pathology and will provide possible opportunities for repair strategies and targeted pharmacotherapy. This review summarizes the lesion components and features, particularly the detrimental elements, and discusses the possibility of suggesting new potential targets as therapies for demyelinating diseases like MS.

Similar content being viewed by others
Data Availability
Not applicable.
References
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA (2018) Multiple sclerosis. Nat Rev Dis Primers 4:43
Giovannoni G, Popescu V, Wuerfel J, Hellwig K, Iacobaeus E, Jensen MB, García-Domínguez JM, Sousa L, De Rossi N, Hupperts R (2022) Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord 15:17562864211066751
Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, Mandrekar J, Bramow S, Metz I, Brück W (2015) Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 78:710–721
Absinta M, Sati P, Schindler M, Leibovitch EC, Ohayon J, Wu T, Meani A, Filippi M, Jacobson S, Cortese IC (2016) Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Investig 126:2597–2609
Plemel JR, Liu W-Q, Yong VW (2017) Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov 16:617
Rayatpour A, Javan M (2021) Targeting the brain lesions using peptides: a review focused on the possibility of targeted drug delivery to multiple sclerosis lesions. Pharmacol Res 167:105441
Rahmanzadeh R, Galbusera R, Lu PJ, Bahn E, Weigel M, Barakovic M, Franz J, Nguyen TD, Spincemaille P, Schiavi S (2022) A new advanced MRI biomarker for remyelinated lesions in multiple sclerosis. Ann Neurol 92:486–502
Homayouny E, Khayati RM, Nabavi SM, Karami V (2022) Perfusion MRI in automatic classification of multiple sclerosis lesion subtypes. IET Signal Process 16(4):377–390
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H (2017) An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 133:13–24
Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354:942–955
Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Stork L, Ellenberger D, Ruprecht K, Reindl M, Beißbarth T, Friede T, Kümpfel T, Gerdes LA, Gloth M, Liman T (2020) Antibody signatures in patients with histopathologically defined multiple sclerosis patterns. Acta Neuropathol 139:547–564
Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I (2018) Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 135:511–528
Allen NJ, Barres BA (2009) Neuroscience: Glia - more than just brain glue. Nature 457:675–677
Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487
Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SE, Couturier CP, Watson BR, Scalisi G, Alkwai S, Rothhammer V (2020) MAFG-driven astrocytes promote CNS inflammation. Nature 578:593–599
Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM (2006) Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53:688–695
Gimsa U, Mitchison NA, Brunner-Weinzierl MC (2013) Immune privilege as an intrinsic CNS property: astrocytes protect the CNS against T-cell-mediated neuroinflammation. Mediators Inflamm 2013:320519
Liddelow S, Hoyer D (2016) Astrocytes: adhesion molecules and immunomodulation. Curr Drug Targets 17:1871–1881
Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR (2012) Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122:2454–2468
Weiner HL (2004) Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol 61:1613–1615
Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J, Fitzgerald KC, Song A, Liu P, Lin J-P (2021) A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature 597:709–714
Silva Oliveira Junior M, Schira-Heinen J, Reiche L, Han S, de Amorim VCM, Lewen I, Gruchot J, Göttle P, Akkermann R, Azim K, Küry P (2022) Myelin repair is fostered by the corticosteroid medrysone specifically acting on astroglial subpopulations. EBioMedicine 2022(83):104204. https://doi.org/10.1016/j.ebiom.2022.104204
Malik O, Compston DA, Scolding NJ (1998) Interferon-beta inhibits mitogen induced astrocyte proliferation in vitro. J Neuroimmunol 86:155–162
Zeinstra E, Wilczak N, Chesik D, Glazenburg L, Kroese FG, De Keyser J (2006) Simvastatin inhibits interferon-gamma-induced MHC class II up-regulation in cultured astrocytes. J Neuroinflammation 3:16
Ghasemi-Kasman M, Zare L, Baharvand H, Javan M (2017) In vivo conversion of astrocytes to myelinating cells by miR-302/367 and valproate to enhance myelin repair. J Tissue Eng Regen Med 12(1):e462–e472
Mokhtarzadeh Khanghahi A, Satarian L, Deng W, Baharvand H, Javan M (2018) In vivo conversion of astrocytes into oligodendrocyte lineage cells with transcription factor Sox10; Promise for myelin repair in multiple sclerosis. PloS one 13:e0203785
Niu W, Zang T, Smith DK, Vue TY, Zou Y, Bachoo R, Johnson JE, Zhang CL (2015) SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep 4:780–794
Zare L, Baharvand H, Javan M (2018) In vivo conversion of astrocytes to oligodendrocyte lineage cells using chemicals: targeting gliosis for myelin repair. Regen Med 13:803–819
Carson MJ (2002) Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia 40:218–231
Strachan-Whaley M, Rivest S, Yong VW (2014) Interactions between microglia and T cells in multiple sclerosis pathobiology. J Interferon Cytokine Res 34:615–622
Saijo K, Glass CK (2011) Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11:775–787
Swanson ME, Murray HC, Ryan B, Faull RL, Dragunow M, Curtis MA (2020) Quantitative immunohistochemical analysis of myeloid cell marker expression in human cortex captures microglia heterogeneity with anatomical context. Sci Rep 10:1–18
Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48(380-395):e386
Sankowski R, Böttcher C, Masuda T, Geirsdottir L, Sindram E, Seredenina T, Muhs A, Scheiwe C, Shah MJ, Heiland DH (2019) Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nature Neurosci 22:2098–2110
Mathys H, Adaikkan C, Gao F, Young JZ, Manet E, Hemberg M, De Jager PL, Ransohoff RM, Regev A, Tsai L-H (2017) Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep 21:366–380
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169(1276-1290):e1217
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J (2019) Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50(253-271):e256
Plemel JR, Stratton JA, Michaels NJ, Rawji KS, Zhang E, Sinha S, Baaklini CS, Dong Y, Ho M, Thorburn K (2020) Microglia response following acute demyelination is heterogeneous and limits infiltrating macrophage dispersion. Sci Adv 6:eaay6324
Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Scheiwe C, Nessler S, Kunz P, van Loo G, Coenen VA (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566:388–392
Beckmann N, Giorgetti E, Neuhaus A, Zurbruegg S, Accart N, Smith P, Perdoux J, Perrot L, Nash M, Desrayaud S (2018) Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol Commun 6:1–17
Nissen JC, Thompson KK, West BL, Tsirka SE (2018) Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery. Exp Neurol 307:24–36
Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, Waisman A, Rülicke T, Prinz M, Priller J (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11:146–152
Ní Gabhann J, Hams E, Smith S, Wynne C, Byrne JC, Brennan K, Spence S, Kissenpfennig A, Johnston JA, Fallon PG (2014) Btk regulates macrophage polarization in response to lipopolysaccharide. PloS one 9:e85834
Bhargava P, Kim S, Reyes AA, Grenningloh R, Boschert U, Absinta M, Pardo C, Van Zijl P, Zhang J, Calabresi PA (2021) Imaging meningeal inflammation in CNS autoimmunity identifies a therapeutic role for BTK inhibition. Brain 144:1396–1408
Groves A, Kihara Y, Chun J (2013) Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci 328:9–18
Choi JW, Gardell SE, Herr DR, Rivera R, Lee C-W, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G (2011) FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci 108:751–756
Jørgensen LØ, Hyrlov K, Elkjaer M, Weber AB, Pedersen A, Svenningsen ÅF, Illes Z (2020) Cladribine modifies functional properties of microglia. Clin Exp Immunol 201:328–340
Aybar F, Perez MJ, Marcora MS, Samman ME, Marrodan M, Pasquini JM, Correale J (2022) 2-Chlorodeoxyadenosine (Cladribine) preferentially inhibits the biological activity of microglial cells. Int Immunopharmacol 105:108571
Musella A, Mandolesi G, Gentile A, Rossi S, Studer V, Motta C, Sepman H, Fresegna D, Haji N, Paolillo A (2013) Cladribine interferes with IL-1β synaptic effects in experimental multiple sclerosis. J Neuroimmunol 264:8–13
Edling A, Woodworth L, Agrawal R, Mahan A, Garron T, Hagan N, Siders B (2017) Teriflunomide impacts primary microglia and astrocyte functions in vitro (P2. 348). In: American Academy of Neurology Annual meeting
Wostradowski T, Prajeeth CK, Gudi V, Kronenberg J, Witte S, Brieskorn M, Stangel M (2016) In vitro evaluation of physiologically relevant concentrations of teriflunomide on activation and proliferation of primary rodent microglia. J Neuroinflammation 13:1–12
Linker RA, Lee D-H, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134:678–692
Pagani F, Testi C, Grimaldi A, Corsi G, Cortese B, Basilico B, Baiocco P, De Panfilis S, Ragozzino D, Di Angelantonio S (2020) Dimethyl fumarate reduces microglia functional response to tissue damage and favors brain iron homeostasis. Neuroscience 439:241–254
Kipp M, Victor M, Martino G, Franklin RJ (2012) Endogeneous remyelination: findings in human studies. CNS Neurol Disord Drug Targets 11:598–609
Chang A, Tourtellotte WW, Rudick R, Trapp BD (2002) Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346:165–173
Franklin RJ, Ffrench-Constant C (2008) Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839–855
Falcão AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, Floriddia EM, Vanichkina DP, Williams A, Guerreiro-Cacais AO (2018) Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med 24:1837–1844
Fernández-Castañeda A, Chappell MS, Rosen DA, Seki SM, Beiter RM, Johanson DM, Liskey D, Farber E, Onengut-Gumuscu S, Overall CC (2020) The active contribution of OPCs to neuroinflammation is mediated by LRP1. Acta Neuropathol 139:365–382
Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J, Strasburger H, Herbst L, Alexis M, Karnell J (2019) Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 10:1–20
Jäkel S, Agirre E, Falcão AM, Van Bruggen D, Lee KW, Knuesel I, Malhotra D, Williams A, Castelo-Branco G (2019) Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566:543–547
Yeung MS, Djelloul M, Steiner E, Bernard S, Salehpour M, Possnert G, Brundin L, Frisén J (2019) Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566:538–542
Heß K, Starost L, Kieran NW, Thomas C, Vincenten MC, Antel J, Martino G, Huitinga I, Healy L, Kuhlmann T (2020) Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol 140:359–375
Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI (2017) Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 14:47
Rajendran L, Bali J, Barr MM, Krämer-Albers E-M, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breakefield XO (2014) Emerging roles of extracellular vesicles in the nervous system. J Neurosci 34:15482–15489
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Fraser KB, Rawlins AB, Clark RG, Alcalay RN, Standaert DG, Liu N, West AB (2016) Ser (P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson's disease. Mov Disord 31:1543–1550
Gomes C, Keller S, Altevogt P, Costa J (2007) Evidence for secretion of Cu, Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett 428:43–46
Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL (2016) Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. The FASEB J 30:3853–3859
Marcos-Ramiro B, Nacarino PO, Serrano-Pertierra E, Blanco-Gelaz MÁ, Weksler BB, Romero IA, Couraud PO, Tuñón A, López-Larrea C, Millán J (2014) Microparticles in multiple sclerosis and clinically isolated syndrome: effect on endothelial barrier function. BMC Neurosci 15:110
Galazka G, Mycko MP, Selmaj I, Raine CS, Selmaj KW (2018) Multiple sclerosis: serum-derived exosomes express myelin proteins. Mult Scler 24(4):449–458. https://doi.org/10.1177/1352458517696597
Pieragostino D, Cicalini I, Lanuti P, Ercolino E, di Ioia M, Zucchelli M, Zappacosta R, Miscia S, Marchisio M, Sacchetta P (2018) Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of multiple sclerosis patients. Sci Rep 8:1–12
Casella G, Rasouli J, Boehm A, Zhang W, Xiao D, Ishikawa LLW, Thome R, Li X, Hwang D, Porazzi P (2020) Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci Transl Med 12:eaba0599
Carandini T, Colombo F, Finardi A, Casella G, Garzetti L, Verderio C, Furlan R (2015) Microvesicles: what is the role in multiple sclerosis? Front Neurol 6:111
Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, Riganti L, Corradini I, Francolini M, Garzetti L, Maiorino C, Servida F, Vercelli A, Rocca M, Dalla Libera D, Martinelli V, Comi G, Martino G, Matteoli M, Furlan R (2012) Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol 72:610–624
Lombardi M, Parolisi R, Scaroni F, Bonfanti E, Gualerzi A, Gabrielli M, de Rosbo NK, Uccelli A, Giussani P, Viani P (2019) Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol 138:987–1012
Dickens AM, Tovar-y-Romo LB, Yoo S-W, Trout AL, Bae M, Kanmogne M, Megra B, Williams DW, Witwer KW, Gacias M (2017) Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal 10:eaai7696
Wang K, Ye L, Lu H, Chen H, Zhang Y, Huang Y, Zheng JC (2017) TNF-α promotes extracellular vesicle release in mouse astrocytes through glutaminase. J Neuroinflammation 14:87
Guo BB, Bellingham SA, Hill AF (2015) The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem 290:3455–3467
Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E (2009) Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO J 28:1043–1054
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
Saenz-Cuesta M, Osorio-Querejeta I, Otaegui D (2014) Extracellular Vesicles in Multiple Sclerosis: What are They Telling Us? Front Cell Neurosci 8:100
Dawson G, Qin J (2011) Gilenya (FTY720) inhibits acid sphingomyelinase by a mechanism similar to tricyclic antidepressants. Biochem Biophys Res Commun 404:321–323
Liu C, Su C (2019) Design strategies and application progress of therapeutic exosomes. Theranostics 9:1015
Karimi-Abdolrezaee S, Schut D, Wang J, Fehlings MG (2012) Chondroitinase and growth factors enhance activation and oligodendrocyte differentiation of endogenous neural precursor cells after spinal cord injury. PloS one 7:e37589
Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB (2005) LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci 8:745–751
Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH (2011) Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci 14:1009–1016
Williams A, Piaton G, Aigrot MS, Belhadi A, Theaudin M, Petermann F, Thomas JL, Zalc B, Lubetzki C (2007) Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain 130:2554–2565
Seyedsadr MS, Weinmann O, Amorim A, Ineichen BV, Egger M, Mirnajafi-Zadeh J, Becher B, Javan M, Schwab ME (2019) Inactivation of sphingosine-1-phosphate receptor 2 (S1PR2) decreases demyelination and enhances remyelination in animal models of multiple sclerosis. Neurobiol Dis 124:189–201
Niknam P, Raoufy MR, Fathollahi Y, Javan M (2019) Modulating proteoglycan receptor PTPσ using intracellular sigma peptide improves remyelination and functional recovery in mice with demyelinated optic chiasm. Mol Cell Neurosci 99:103391
Syed YA, Baer A, Hofer MP, Gonzalez GA, Rundle J, Myrta S, Huang JK, Zhao C, Rossner MJ, Trotter MW, Lubec G, Franklin RJ, Kotter MR (2013) Inhibition of phosphodiesterase-4 promotes oligodendrocyte precursor cell differentiation and enhances CNS remyelination. EMBO Mol Med 5:1918–1934
Mi S, Lee X, Hu Y, Ji B, Shao Z, Yang W, Huang G, Walus L, Rhodes K, Gong BJ, Miller RH, Pepinsky RB (2011) Death receptor 6 negatively regulates oligodendrocyte survival, maturation and myelination. Nat Med 17:816–821
Carbajal KS, Miranda JL, Tsukamoto MR, Lane TE (2011) CXCR4 signaling regulates remyelination by endogenous oligodendrocyte progenitor cells in a viral model of demyelination. Glia 59:1813–1821
Zhang J, Cheng Y, Cui W, Li M, Li B, Guo L (2014) MicroRNA-155 modulates Th1 and Th17 cell differentiation and is associated with multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol 266:56–63
Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G (2009) MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 10:1252–1259
Gandhi R, Healy B, Gholipour T, Egorova S, Musallam A, Hussain MS, Nejad P, Patel B, Hei H, Khoury S, Quintana F, Kivisakk P, Chitnis T, Weiner HL (2013) Circulating microRNAs as biomarkers for disease staging in multiple sclerosis. Ann Neurol 73:729–740
Shen Q, Temple S (2009) Fine control: microRNA regulation of adult neurogenesis. Nat Neurosci 12:369–370
Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R, Meinl E (2009) MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 132:3342–3352
Moore CS, Rao VT, Durafourt BA, Bedell BJ, Ludwin SK, Bar-Or A, Antel JP (2013) miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann Neurol 74:709–720
Juźwik CA, Drake SS, Zhang Y, Paradis-Isler N, Sylvester A, Amar-Zifkin A, Douglas C, Morquette B, Moore CS, Fournier AE (2019) microRNA dysregulation in neurodegenerative diseases: a systematic review. Prog Neurobiol 182:101664
Wu T, Chen G (2016) miRNAs participate in MS pathological processes and its therapeutic response. Mediators Inflamm 2016:4578230
Fu R, Shen Q, Xu P, Luo JJ, Tang Y (2014) Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol 49:1422–1434
Mandolesi G, De Vito F, Musella A, Gentile A, Bullitta S, Fresegna D, Sepman H, Di Sanza C, Haji N, Mori F (2017) miR-142-3p is a key regulator of IL-1β-dependent synaptopathy in neuroinflammation. J Neurosci 37:546–561
Lescher J, Paap F, Schultz V, Redenbach L, Scheidt U, Rosewich H, Nessler S, Fuchs E, Gartner J, Bruck W, Junker A (2012) MicroRNA regulation in experimental autoimmune encephalomyelitis in mice and marmosets resembles regulation in human multiple sclerosis lesions. J Neuroimmunol 246:27–33
Rao VTS, Fuh SC, Moore CS, Ludwin SK, Ho MK, Bedell BJ, Bar-Or A, Antel JP (2014) Expression profiles of inflammation associated microRNAs in astrocytes from multiple sclerosis lesions (Abstract, P620). In: Proceedings of the 2014 Joint ECTRIMS-ACTRIMS meeting (MSBoston 2014). Multiple Sclerosis Journal 20(S1):285–496
Ingram G, Hakobyan S, Hirst CL, Harris CL, Pickersgill TP, Cossburn MD, Loveless S, Robertson NP, Morgan BP (2010) Complement regulator factor H as a serum biomarker of multiple sclerosis disease state. Brain 133:1602–1611
Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ (2010) Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem 285:38951–38960
Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8:500–508
Heidenreich KA, Linseman DA (2004) Myocyte enhancer factor-2 transcription factors in neuronal differentiation and survival. Mol Neurobiol 29:155–166
Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, Soussou W, Nie Z, Kang YJ, Nakanishi N, Okamoto S, Roberts AJ, Schwarz JJ, Lipton SA (2008) Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci U.S.A 105:9397–9402
Wilker PR, Kohyama M, Sandau MM, Albring JC, Nakagawa O, Schwarz JJ, Murphy KM (2008) Transcription factor Mef2c is required for B cell proliferation and survival after antigen receptor stimulation. Nature immunology 9:603–612
Fu W, Wei J, Gu J (2006) MEF2C mediates the activation induced cell death (AICD) of macrophages. Cell Res 16:559–565
Bhat NR, Zhang P, Mohanty SB (2007) p38 MAP kinase regulation of oligodendrocyte differentiation with CREB as a potential target. Neurochem Res 32:293–302
Deczkowska A, Matcovitch-Natan O, Tsitsou-Kampeli A, Ben-Hamo S, Dvir-Szternfeld R, Spinrad A, Singer O, David E, Winter DR, Smith LK, Kertser A, Baruch K, Rosenzweig N, Terem A, Prinz M, Villeda S, Citri A, Amit I, Schwartz M (2017) Mef2C restrains microglial inflammatory response and is lost in brain ageing in an IFN-I-dependent manner. Nat Commun 8:717
Han MH, Hwang SI, Roy DB, Lundgren DH, Price JV, Ousman SS, Fernald GH, Gerlitz B, Robinson WH, Baranzini SE, Grinnell BW, Raine CS, Sobel RA, Han DK, Steinman L (2008) Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451:1076–1081
Riederer BM (2007) Microtubule-associated protein 1B, a growth-associated and phosphorylated scaffold protein. Brain Res Bull 71:541–558
Dangond F, Donnelly A, Hohlfeld R, Lubetzki C, Kohlhaas S, Leocani L, Ciccarelli O, Stankoff B, Sormani MP, Chataway J (2021) Facing the urgency of therapies for progressive MS—a Progressive MS Alliance proposal. Nat Rev Neurol 17:185–192
Funding
This study was supported by grants from Royan Institute (grant number #2100) and Tarbiat Modares University (grant number no. 310860) provided to M.J.
Author information
Authors and Affiliations
Contributions
AMK and AR drafted the first version of the paper; HB and MJ revised and edited the paper; MJ raised up the concept and approved the final version of the paper.
Corresponding author
Ethics declarations
Ethical approval
None
Informed consent
All authors were consent to publish.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mokhtarzadeh Khanghahi, A., Rayatpour, A., Baharvand, H. et al. Neuroglial components of brain lesions may provide new therapeutic strategies for multiple sclerosis. Neurol Sci 44, 3795–3807 (2023). https://doi.org/10.1007/s10072-023-06915-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06915-5