Abstract
Background
Observational studies have found a significant association between smoking and smaller gray matter volume, but this finding was limited by the reverse causality bias and possible confounding factors. Therefore, we conducted a Mendelian randomization (MR) study to explore the causal association of smoking with brain gray and white matter volume from a genetic perspective, and to investigate the possible mediators influencing the association.
Methods
Smoking initiation (ever being a regular smoker) was used as the primary exposure from the GWAS & Sequencing Consortium of Alcohol and Nicotine use in up to 1,232,091 individuals of European descent. Their associations with brain volume were acquired from a recent genome-wide association study of brain imaging phenotypes conducted among 34,298 individuals of the UK Biobank. The random-effects inverse-variance weighted method was applied as the main analysis. Multivariable MR analysis was performed to assess the potential interference of confounding factors on causal effect.
Results
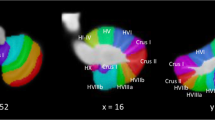
Genetic liability to smoking initiation was significantly associated with lower gray matter volume (beta, −0.100; 95% CI, −0.156 to −0.043; P=5.23×10-4) but not with white matter volume. Multivariable MR results suggested that the association with lower gray matter volume might be mediated by alcohol drinking. Regarding localized gray matter volume, genetic liability to smoking initiation was associated with lower gray matter volume in left superior temporal gyrus, anterior division and right superior temporal gyrus, posterior division.
Conclusions
This MR study supports the association between smoking and lower gray matter volume, and highlights the importance of never smoking.


Similar content being viewed by others
Data availability
All data generated or analyzed in this study are available in the supplementary material or associated publicly GWAS dataset.
References
GBD 2019 Tobacco Collaborators (2021) Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397(10292):2337–2360. https://doi.org/10.1016/s0140-6736(21)01169-7
Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K et al (2014) Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology 39(11):2594–2600. https://doi.org/10.1038/npp.2014.112
Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J et al (2019) Mega-analysis of gray matter volume in substance dependence: general and substance-specific regional effects. Am J Psychiatry 176(2):119–128. https://doi.org/10.1176/appi.ajp.2018.17040415
CCox SR, Lyall DM, Ritchie SJ, Bastin ME, Harris MA, Buchanan CR et al (2019) Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J 40(28):2290–2300. https://doi.org/10.1093/eurheartj/ehz100
Elbejjani M, Auer R, Jacobs DR Jr, Haight T, Davatzikos C, Goff DC Jr et al (2019) Cigarette smoking and gray matter brain volumes in middle age adults: the CARDIA Brain MRI sub-study. Transl Psychiatry 9(1):78. https://doi.org/10.1038/s41398-019-0401-1
Gray JC, Thompson M, Bachman C, Owens MM, Murphy M, Palmer R (2020) Associations of cigarette smoking with gray and white matter in the UK Biobank. Neuropsychopharmacology 45(7):1215–1222. https://doi.org/10.1038/s41386-020-0630-2
Linli Z, Rolls ET, Zhao W, Kang J, Feng J, Guo S (2023) Smoking is associated with lower brain volume and cognitive differences: a large population analysis based on the UK Biobank. Prog Neuro-Psychopharmacol Biol Psychiatry 123:110698. https://doi.org/10.1016/j.pnpbp.2022.110698
Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F et al (2019) Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 51(2):237–244. https://doi.org/10.1038/s41588-018-0307-5
Daviet R, Aydogan G, Jagannathan K, Spilka N, Koellinger PD, Kranzler HR, Nave G et al (2022) Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat Commun 13(1):1175. https://doi.org/10.1038/s41467-022-28735-5
Larsson SC, Mason AM, Bäck M, Klarin D, Damrauer SM, Michaëlsson K et al (2020) Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J 41(35):3304–3310. https://doi.org/10.1093/eurheartj/ehaa193
Park S, Lee S, Kim Y, Cho S, Kim K, Kim YC et al (2021) Causal effects of atrial fibrillation on brain white and gray matter volume: a Mendelian randomization study. BMC Med 19(1):274. https://doi.org/10.1186/s12916-021-02152-9
Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA et al (2016) Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct 12(1):16. https://doi.org/10.1186/s12993-016-0100-5
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27(8):1133–1163. https://doi.org/10.1002/sim.3034
Myers TA, Chanock SJ, Machiela MJ (2020) LDlinkR: An R package for rapidly calculating linkage disequilibrium statistics in diverse populations. Front Genet 11:157. https://doi.org/10.3389/fgene.2020.00157
Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ et al (2020) Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med 50(14):2435–2443. https://doi.org/10.1017/s0033291719002678
Burgess S, Thompson SG (2011) Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40(3):755–764. https://doi.org/10.1093/ije/dyr036
Smith SM, Douaud G, Chen W, Hanayik T, Alfaro-Almagro F, Sharp K et al (2021) An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat Neurosci 24(5):737–745. https://doi.org/10.1038/s41593-021-00826-4
Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ et al (2021) Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ 375:n2233. https://doi.org/10.1136/bmj.n2233
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37(7):658–665. https://doi.org/10.1002/gepi.21758
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2):512–525. https://doi.org/10.1093/ije/dyv080
Zhao Q, Wang J, Hemani G, Bowden J, Small DS (2020) Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Stat 48(3):1742–1769. https://doi.org/10.1214/19-AOS1866
Verbanck M, Chen CY, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698. https://doi.org/10.1038/s41588-018-0099-7
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40(4):304–314. https://doi.org/10.1002/gepi.21965
Greco MF, Minelli C, Sheehan NA, Thompson JR (2015) Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med 34(21):2926–2940. https://doi.org/10.1002/sim.6522
Burgess S, Thompson SG (2015) Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 181(4):251–260. https://doi.org/10.1093/aje/kwu283
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR et al (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518(7538):197–206. https://doi.org/10.1038/nature14177
Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M et al (2018) Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 50(8):1112–1121. https://doi.org/10.1038/s41588-018-0147-3
Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE et al (2018) Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet 50(9):1234–1239. https://doi.org/10.1038/s41588-018-0171-3
Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A et al (2018) Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 50(4):524–537. https://doi.org/10.1038/s41588-018-0058-3
Linli Z, Feng J, Zhao W, Guo S (2022) Associations between smoking and accelerated brain ageing. Prog Neuro-Psychopharmacol Biol Psychiatry 113:110471. https://doi.org/10.1016/j.pnpbp.2021.110471
Swan GE, Lessov-Schlaggar CN (2007) The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 17(3):259–273. https://doi.org/10.1007/s11065-007-9035-9
Rabinowitz JA, Campos AI, Ong JS, García-Marín LM, Alcauter S, Mitchell BL et al (2022) Shared genetic etiology between cortical brain morphology and tobacco, alcohol, and cannabis use. Cereb Cortex 32(4):796–807. https://doi.org/10.1093/cercor/bhab243
Weng JC, Chuang YC, Zheng LB, Lee MS, Ho MC (2022) Assessment of brain connectome alterations in male chronic smokers using structural and generalized q-sampling MRI. Brain Imaging Behav 16(4):1761–1775. https://doi.org/10.1007/s11682-022-00647-4
Liu H, Guan L, Nie Y, Li Q, Xue J, Yang Y et al (2022) Brain magnetic resonance imaging features of nicotine-dependent individuals and its correlation with polymorphisms of dopamine d receptor gene. Contrast Media Mol Imaging 2022:2296776. https://doi.org/10.1155/2022/2296776
Logtenberg E, Overbeek MF, Pasman JA, Abdellaoui A, Luijten M, van Holst RJ et al (2022) Investigating the causal nature of the relationship of subcortical brain volume with smoking and alcohol use. Br J Psychiatry 221(1):377–385. https://doi.org/10.1192/bjp.2021.81
Topiwala A, Ebmeier KP, Maullin-Sapey T, Nichols TE (2022) Alcohol consumption and MRI markers of brain structure and function: cohort study of 25,378 UK Biobank participants. Neuroimage Clin 35:103066. https://doi.org/10.1016/j.nicl.2022.103066
Carmody TP, Brischetto CS, Matarazzo JD, O'Donnell RP, Connor WE (1985) Co-occurrent use of cigarettes, alcohol, and coffee in healthy, community-living men and women. Health Psychol 4(4):323–335. https://doi.org/10.1037//0278-6133.4.4.323
Debette S, Markus HS (2010) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341:c3666. https://doi.org/10.1136/bmj.c3666
Taylor-Bateman V, Gill D, Georgakis M, Malik R, Munroe P, Traylor M et al (2022) Cardiovascular risk factors and MRI markers of cerebral small vessel disease: a Mendelian randomization study. Neurology 98(4). https://doi.org/10.1212/WNL.0000000000013120
Acknowledgements
We thank all the participants and staff of the GWAS dataset we used.
Author information
Authors and Affiliations
Contributions
Wenjuan Lin and Yunlong Lu contributed to the concept and design of the work. Wenjuan Lin and Lisheng Zhu contributed to the data processing, integrative analyses, writing manuscript, and generating figures and tables. Yunlong Lu critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study is based on publicly available data sources, and no identifiable patient data were collected. Ethical approval and informed consent were available in the original genomic studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Tables S1–S6 (PDF 1254 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, W., Zhu, L. & Lu, Y. Association of smoking with brain gray and white matter volume: a Mendelian randomization study. Neurol Sci 44, 4049–4055 (2023). https://doi.org/10.1007/s10072-023-06854-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06854-1