Abstract
Background
Ticagrelor is one of the most recent antiplatelet drugs to be approved to treat ischemic heart disease. Its efficacy may exceed aspirin in improving clinical outcomes in patients with acute ischemic stroke who are ineligible for rt-PA.
Objectives
We evaluated the safety regarding hemorrhagic complications (as a primary endpoint) and the efficacy (as a secondary endpoint) of a 180-mg loading dose of ticagrelor given within 9 h from the onset of the first-ever non-cardioembolic ischemic stroke.
Methods
We conducted our study on patients aged 18–75 years who presented with their first clinically manifested non-cardioembolic ischemic stroke and were recruited from the emergency department OF Kafr El-Sheik University Hospitals, Egypt.
Eligible patients randomly received ticagrelor or aspirin loading and maintenance doses. Screening, randomization, and initiation of treatment all occurred within the first 9 h of stroke onset.
Results
Eighty-five patients received ticagrelor, and 84 received aspirin. Patients who received ticagrelor had a better clinical outcome in terms of NIHSS improvement at 2 days and 1 week of discharge and a favorable mRS score after 1 week of discharge and at 90-day follow-up. There was no significant difference between the two groups regarding hemorrhagic adverse effects.
Conclusion
This pilot study found that ticagrelor had a better clinical outcome than aspirin based on NIHSS and mRS in acute ischemic stroke patients who received it within 9 h from symptom onset and had a shorter hospital stay duration. Ticagrelor was non-inferior to aspirin regarding hemorrhagic complications.
Trial registration
We registered our trial on ClinicalTrials.gov, named after “ticagrelor versus aspirin in ischemic stroke,” and with a clinical trial number (NCT03884530)—March 21, 2019.
Similar content being viewed by others
Background
Stroke is one of the leading causes of long-term disability. It is the second most typical cause of mortality globally. The burden of stroke is much higher in developing countries, accounting for 66% of worldwide strokes [1].
rt-PA is still the only approved treatment for acute stroke. Nevertheless, its accessibility is still low in not only developing countries but also high-income countries due to derangements in supply chains related to war and lack of materials. For this reason, there is an ongoing need for using a loading dose of new antiplatelet agents, which may provide better results for acute stroke patients who are ineligible for rt-PA [2].
Ticagrelor is a new antiplatelet medication that acts by reversible blockade of the P2Y12 subtype of adenosine diphosphate (ADP) receptor. It was approved by the US Food and Drug Administration (FDA) as a treatment for acute coronary syndromes in 2011, and in 2015, it received approval as a maintenance treatment in patients with a history of a heart attack [3, 4], and in 2020 it was approved by FDA in ischemic stroke secondary prevention based on the results of the THALES trial [5].
Our trial differs from other ticagrelor studies as it focused on comparing the safety and efficacy of ticagrelor versus aspirin within the first 9 h of a first-ever ischemic stroke by assessing the early change in NIHSS after 2 days and 1 week and the change in mRS after 1 week and 3 months, we started the antiplatelet treatment within 9 h of onset, as the ischemic penumbra may remain viable for up to 12 h [6].
Along with the current clinical trial, the efficacy and safety of 180-mg loading dose of ticagrelor administered within 9 h of first-ever ischemic stroke compared to aspirin were assessed through NIHSS, mRS, duration of hospital stay, and possible hemorrhagic complications.
Methods
Sample size
This pilot study was based on the study carried out by Johnston and colleagues (2016) [7]. We used Epi Info STATCALC to calculate the sample size by considering the following assumptions: 95% two-sided confidence level, with a power of 80%, alpha error of 5%, and odds ratio calculated = 1.115. The final maximum sample size, which was taken from the Epi Info output, was 156. We increased the sample size to 169 patients to assume any dropout cases during the follow-up period. A total of 169 patients continued to follow up along the entire study timeline, of which 85 patients received ticagrelor and 84 patients received aspirin.
We conducted our open-label randomized prospective controlled trial between May 2019 and September 2020 after approval of the ethical committee of the faculty of medicine at Ain Shams University and the ethical committee of the faculty of medicine Kafr El-Sheik University.
We got formal written informed consent from all eligible patients or their first order of kin before randomization.
We used a web-based centralized blocked randomization plan to randomly allocate patients in a one-to-one ratio to receive ticagrelor or aspirin. Still, all of the clinical investigators were blind to the block size of the randomization plan, but the patients were aware of the antiplatelet used in the study.
The study was composed of two parallel groups: the ticagrelor group, which received a 180 mg loading dose during the first 9 h of stroke onset followed by 90 mg b.i.d from the second to the 90th day, and the aspirin group, which received a 300 mg loading dose during the first 9 h of stroke onset followed by 300 mg q.d. from the 2nd day to the 14th day then 75 mg q.d. from the 15th day to the 90th day.
This analysis was specifically designed as a pilot study to examine the preliminary efficacy, safety, and feasibility of pursuing a large-scale randomized clinical trial powered properly for safety and efficacy.
Inclusion criteria
We included both genders with eligible ages ranging between 18 and 75 years, with the first-ever presentation with acute non-cardioembolic ischemic stroke diagnosed by appropriate clinical history, examination, and specific brain imaging findings. Patients with previous transient ischemic attacks (TIA) were not excluded from the study. Patients are not eligible for rt-PA treatment, as rt-PA might lead to hemorrhagic complications and bias our assessment of the safety of the trial medications. Also, In patients who are treated with rt-PA, initiation of antiplatelet agents should be delayed until after 24 h post-thrombolysis [8].
Exclusion criteria
We excluded patients who had not been followed up on for 90 days after enrollment, those with NIHSS less than three or more than 25 or who had rapidly resolving symptoms before imaging results, patients with a known history of persistent or recurrent CNS pathology (e.g., epilepsy, meningioma, multiple sclerosis, history of head trauma with a residual neurological deficit).
We excluded patients who had a cardioembolic ischemic stroke before starting treatment or retrogradely. We considered ischemic stroke a cardio-embolic one when the patient had major or minor risk factors of having a cardiac source of embolus as mechanical cardiac valves, atrial fibrillation, mitral valve prolapse, aortic valve stenosis or calcification, and patent foramen ovale [9, 10], and we considered the patient to have clinical AF when his standard 12-lead ECG recording had at least 30 s showing heart rhythm with no discernible repeating P waves and irregular RR intervals (when atrioventricular conduction is not impaired) [11].
We ruled out patients who had already recurrent stroke before enrollment in our trial diagnosed by appropriate clinical history and/or MRI brain findings, as those patients might receive antiplatelet treatment as secondary prevention and might have a residual neurological deficit which could bias the assessment of safety and efficacy of the antiplatelet in our trial.
We excluded patients who had clinical seizures at the onset of their stroke, as well as those who had symptoms of any major organ failure, active malignancies, or an acute myocardial infarction within the previous 6 weeks, and those who were on warfarin or new oral anticoagulants, regular ticagrelor during the week before admission, or chemotherapy within the previous year.
For safety measures and to avoid associated confounders, we excluded patients with active peptic ulcers, GIT surgery, bleeding history within the last year, and those with a history of major surgery within the last 3 months.
We ruled out of our trial patients who had a known allergy to the study drugs and those with INR > 1.4, P.T. > 18, blood glucose level < 50, > 400 mg/DL, blood pressure < 90/60, > 185/110 mmHg on admission, or platelets < 100,000.
We considered pregnant and lactating patients or those with stroke due to venous thrombosis and stroke following cardiac arrest, or profuse hypotension ineligible for our trial.
Study procedures
We collected the following data: age, sex, medical history of hypertension (HTN), diabetes mellitus (DM), ischemic heart disease (IHD), hyperlipidemia, tobacco use, and the time from symptoms onset to the start of treatment.
We diagnosed ischemic stroke based on the specific clinical history and examination and MRI brain using stroke protocol: T1W, T2W, FLAIR, DWI, T2 echo gradient, and MRA of all intra-cerebral vessels.
Before randomization, all the patients assessed for eligibility to participate in the trial underwent 12-lead routine ECG and transthoracic echocardiography, and immediately after randomization and receiving loading antiplatelet treatment, every patient enrolled in our trial underwent 24 h of continuous cardiac rhythm monitoring and transesophageal echocardiography, and we excluded patients with major or minor risk factors for cardio-embolic stroke.
All the patients in our study underwent carotid duplex and baseline laboratory investigations (lipid profile, liver functions, coagulation profile, complete blood count, and blood sugar).
We estimated the safety of loading ticagrelor by looking at hemorrhagic complications assessed using the PLATO bleeding definition [12].
Hemorrhagic transformation of the infarct was determined by performing a follow-up CT brain scan after 2 days and after 1 week or discharge to detect the hemorrhage; additionally, the European Cooperative Acute Stroke Study (ECASS) classification [13] was used to detect the type of hemorrhagic transformation.
We evaluated clinical improvement using five factors: the first two factors assessed the difference between NIHSS at baseline and the 2nd day and the difference between NIHSS at baseline and (the 7th day of discharge) in each patient. A decrease of four points or more in the NIHSS score was considered a significant improvement [14].
The third and fourth factors assessed mRS after 1 week of discharge and after 90 days, and all of our patients had baseline mRS of zero. mRS ≤ 2 was considered a favorable outcome [15, 16].
The fifth factor was the total number of days each patient spent in the hospital.
Primary endpoint
Safety of ticagrelor (180 mg loading dose during the first 9 h of stroke onset followed by 90 mg twice daily from the 2nd to the 90th day given orally) vs aspirin (300 mg loading dose during the first 9 h of stroke onset followed by 300 mg q.d. from the 2nd day to the 14th day then 75 mg q.d. from the 15th day to the 90th day) regarding hemorrhagic complications using the PLATO bleeding definition, and was assessed by rates of patient suffered from hemorrhagic complications in each group.
Secondary endpoint
The clinical outcome of the patients (assessed by rates of a favorable outcome with [NIHSS] decrease by 4 points or more on the 2nd day of admission and the 7th day of discharge compared to baseline), and (rates of a favorable outcome with mRS 0–2 after 1 week or discharge and after 90 days in a face-to-face interview in the outpatient clinic).
Statistical analysis of the data
All efficacy and safety analyses were based on the intention-to-treat principle, and we analyzed our data using the IBM SPSS software package, version 20.0 (Armonk, NY: IBM Corp.). Statistical analysis was performed for the primary and secondary endpoints separately.
We described numerical data as means ± S.D. or median and interquartile range [IQR]), depending on their distribution, assessed by the Shapiro–Wilk test, and we expressed categorical data using numbers and percent.
We used Pearson’s chi-square to correlate categorical data and the Mann–Whitney U test to compare the abnormally distributed numerical data.
Results
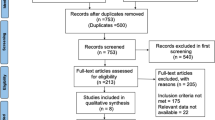
Overall, 650 patients were screened for eligibility; 200 patients (132 males and 68 females) underwent randomization and were divided into two parallel groups. The aspirin group consisted of 99 patients, and the ticagrelor group consisted of 101 patients; a total of 169 patients (113 males and 56 females) completed the pilot study during the 3-month follow-up period, as shown in Fig. 1.
There were no statistically significant differences between the two study arms regarding the baseline characters, as shown in Table 1.
Hemorrhagic transformation of infarction inflicted two patients (2%) in the aspirin group; one had ECASS HI type 1. The other had ECASS HI type 2. In comparison, in the ticagrelor group, one patient (1%) had hemorrhagic infarction (ECASS HI type 1). Minor bleeding (hematuria) occurred in two patients (2%) in the aspirin group and three patients (3%) in the ticagrelor group, with no statistically significant differences between the two groups, as shown in Table 2.
Regarding clinical outcomes, there were no statistically significant differences between the two study arms regarding the decrease in NIHSS score after 2 days, but in the ticagrelor arm, 58 (57.4%) patients showed significant improvement regarding the decrease in NIHSS score after 1 week or discharge compared with 35 (35.4%) in the aspirin group with a P-value (< 0.01), as shown in Table 3.
Regarding mRS, 27 (26.7%) and 39 (38.6%) patients in the ticagrelor group showed favorable mRS scores after 1 week or discharge and after 90 days, respectively, compared with 14 (14.1%), 23 (23.2%) in the aspirin group with P-value (0.04 and 0.02), as shown in Table 3.
Discussion
There is an increasing need for new antiplatelets with better efficacy in managing ischemic stroke patients, especially those who do not fit rt-PA therapy secondary to its relatively short window (4.5 h of symptom onset) and its relatively high price and low accessibility, especially in developing countries. [2].
Regarding the current clinical trial’s primary endpoint, there was no statistically significant difference between the two groups regarding hemorrhagic complications. This result agrees with the results of Johnston and colleagues (2016), Wang and colleagues (2017), and Amarenco and colleagues (2017), who found that there was no statistically significant difference between ticagrelor and aspirin as regards hemorrhagic infarction [7, 17, 18].
Concerning our secondary endpoint, we found that patients who received ticagrelor showed statistically significant clinical improvement regarding NIHSS score after 2 days and 1 week of discharge and more favorable mRS scores after 1 week of discharge and after 90 days compared with patients who received aspirin.
Even though there has been no such study that compared the outcome of ischemic stroke in patients receiving, loading ticagrelor, or loading aspirin within the first 9 h of onset, our results can be partially in agreement with Wang and colleagues (2017), Amarenco and colleagues (2017), and Johnston and colleagues (2020), who found that patients who received ticagrelor had better clinical outcomes than those receiving aspirin results [5, 17, 18].
Our results may be that ticagrelor is a potent P2Y12 adenosine diphosphate platelet receptor inhibitor that decreases platelet activation in the acute phase of ischemic stroke, which prevents the release of neurotoxic and thrombogenic eicosanoids, including thromboxane B2, and, as a result, it might reduce early death and improve outcomes in survivors by reducing the volume of brain penumbra and reducing the risk of early recurrent ischemic stroke and pulmonary embolism Van Kotte and colleagues (1994) and Wallentin and colleagues (2009) [19, 20].
Although our results were encouraging, our pilot study has some limitations: first, the sample was intended to establish the feasibility of a larger-scale trial powered for both safety and efficacy, and this nature of the sample limits the validity and generalizability of the result; second, patients were not blind to the treatment they received.
Conclusion
Compared to aspirin, ticagrelor had a better clinical outcome based on NIHSS and mRS on acute ischemic stroke patients who received it within 9 h from symptom onset and had a less hospital stay duration. Ticagrelor was non-inferior to aspirin regarding hemorrhagic complications.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to the ethical regulations of our university but are available from the corresponding author (Mohamed G. Zeinhom) on reasonable request.
Abbreviations
- ADP :
-
Adenosine diphosphate
- CNS:
-
Central nervous system
- CT :
-
Computerized tomography
- ECASS :
-
European cooperative acute stroke study
- ECG :
-
Electrocardiogram
- FDA :
-
Food and Drug Administration
- GIT :
-
Gastrointestinal tract
- INR :
-
International normalization ratio
- IQR :
-
Interquartile range
- MRI :
-
Magnetic resonance imaging
- mRS :
-
Modified Rankin scale
- NIHSS :
-
National Institute of Health Stroke Score
- rTPA :
-
Recombinant tissue plasminogen activator
- TIA :
-
Transient ischemic attack
References
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367(9524):1747–1757
Meyer DM, Albright KC, Allison TA, Grotta JC (2008) LOAD: a pilot study of the safety of loading of aspirin and clopidogrel in acute ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 17(1):26–29
Jacobson KA, Boeynaems JM (2010) P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today 15(13–14):570–8. https://doi.org/10.1016/j.drudis.2010.05.011
Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Held P et al (2015) Acute stroke or transient ischemic attack treated with aspirin or ticagrelor and patient outcomes (SOCRATES) trial: rationale and design. Int J Stroke 10(8):1304–1308
Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S et al (2020) Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med 383(3):207–217
Paciaroni M, Caso V, Agnelli G (2009) The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol 61(6):321–330
Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR et al (2016) Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 375(1):35–43
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American. Stroke A 50:344–418
Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ et al (2014) Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 13(4):429–438. https://doi.org/10.1016/S1474-4422(13)70310-7
Ntaios G, Hart RG (2017) Embolic stroke. Circulation 136(25):2403–2405
Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GA et al (2021) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 42(5):373–498
Schulman S, Anger SU, Bergqvist D, Eriksson B, Lassen MR, Fisher W (2010) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 8(1):202–204
Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D et al (2011) New England J. 1317–29.
Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W et al (2007) Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): an observational study. Lancet 369(9558):275–282
Furlan AJ (2002) Acute stroke trials: strengthening the underpowered. Stroke 33(6):1450–1451
Broderick JP, Lu M, Kothari R, Levine SR, Lyden PD, Haley EC et al (2000) Finding the most powerful measures of the effectiveness of tissue plasminogen activator in the NINDS tPA stroke trial. Stroke 31(10):2335–2341
Wang Y, Minematsu K, Wong KSL, Amarenco P, Albers GW, Denison H et al (2017) Ticagrelor in acute stroke or transient ischemic attack in Asian patients. Stroke 48(1):167–173
Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P et al (2017) Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 16(4):301–10. https://doi.org/10.1016/S1474-4422(17)30038-8
Van KF, Ciabattoni G, Patrono C, Schmitz PIM, Van GJ, Koudstaal PJ (1994) Evidence for episodic platelet activation in acute ischemic stroke. Stroke 25(2):278–281
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C et al (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361(11):1045–1057. https://doi.org/10.1056/NEJMoa0904327
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Dr. Zeinhom was the principal investigator who collected data, and Prof. Hany Aref was the main supervisor of the study. Profs. El-Khawas and Elbassiouny shared in the study plan revision and supervision, while Profs. Shokri and Roushdy shared in the data analysis and manuscript writing.
Corresponding author
Ethics declarations
Ethical approval
Our study had the approval of the ethical committee of Ain Shams University, and the ethical reference number is (FMASU: MD 88/2019). All methods of our trial were performed in accordance with the guidelines and regulations of the Faculty of Medicine Ain Shams University (FMASU), which is organized and operated according to the guidelines of the International Council on Harmonization (ICH), Anesthesiology and the Islamic Organization for Medical Service (IOMS), the United States Office for Human Research Protections, and the United States Code of Federal Regulations, and operates under Federal Wide Assurance no (FWA00001785).
Consent to participate
Before randomization, formal written informed consent was obtained from the included patients or their first-degree relatives.
Consent for publications
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aref, H.M., El-Khawas, H., Elbassiouny, A. et al. A randomized pilot study of the efficacy and safety of loading ticagrelor in acute ischemic stroke. Neurol Sci 44, 765–771 (2023). https://doi.org/10.1007/s10072-022-06525-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06525-7