Abstract
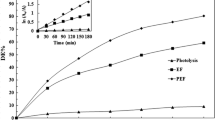
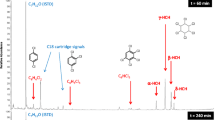
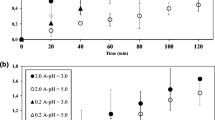
Degradation of the pesticides fenothiocarb and imidacloprid in water using contact glow discharge electrolysis (CGDE) achieved rates of 57.8 and 43.2% respectively. Degradation was enhanced using citric or hydrochloric acid to lower the pH to 3.0. Both acids enhanced both the degradation rate and the amount. Fenothiocarb degradation was 77.14% at pH 3 with citric acid, and 100% with HCl. Degradation of imidacloprid at pH 3 was 70.18% with citric acid and 93.02% with HCl. Acidic conditions favor either production of ·OH radicals or enhancement of the degradation of organic compounds by ·OH. Both the degradation rates and amounts for fenothiocarb and imidacloprid were reduced in the presence of methanol due to competition between pesticides and MeOH for ·OH. Degradation of pesticides was not completely inhibited by MeOH. Degradation of both fenothiocarb and imidacloprid using CGDE obeys a first-order rate law with high regression coefficient values (R 2>0.99).
Similar content being viewed by others
References
Min ZW, Lee JY, Son KA, Im GJ, Hong SM. Development and validation of a quick easy cheap effective rugged and safe-based multi-residues analysis method for persimmon, grape and pear using liquid chromatography-tandem mass spectrometry. J. Korean Soc. Appl. Biol. Chem. 54: 771–777 (2011)
Min ZW, Kim TH, Sin JH, Lee SM Kim JE. Accelerated effect of ferric salts on degradation of thiophosphate fungicide, tolclofosmethyl by zerovalent iron. J. Korean Soc. Appl. Biol. Chem. 52: 681–687 (2009)
Min ZW, Jeon YH Kim JE. Degradation of thiophosphate fungicide, tolclofos-methyl by calcium hydroxide and zerovalent iron in soil. J. Korean Soc. Appl. Biol. Chem. 54: 568–574 (2011)
Glass BL. Relationship between the degradation of DDT and iron redox system in soils. J. Agr. Chem. 20: 324–327 (1972)
Hofstetter TB, Schwarzenbach RP Haderlein SB. Reactivity of Fe (II) species associated with clay minerals. Environ. Sci. Technol. 37: 519–528 (2003)
Ghauch A, Suptil J. Remediation of s-triazines contaminated water in a laboratory scale apparatus using zerovalent iron powder. Chemosphere 41: 1835–1843 (2000)
Satapanajaru T, Comfort SD Shea PJ. Enhancing metolachlor destruction rates with aluminum and iron salts during zerovalent iron treatment. J. Environ. Qual. 32: 1726–1734 (2003)
Ikehata K, El-Din MG. Aqueous pesticide degradation by ozonation and ozone-based advanced oxidation process-A review (Part 1). Ozone. Sci. Eng. 27: 83–114 (2003)
Reynolds G, Graham N, Perry R Rice RG. Aqueous ozonation of pesticides-A review. Ozone. Sci. Eng. 11: 339–382 (1989)
Rice RG. Applications of ozone for industrial wastewater treatment- A review. Ozone. Sci. Eng. 18: 477–515 (1997)
Wu C, Linden KG. Degradation and byproduct formation of parathion in aqueous solutions by UV and UV/H2O2 treatment. Water Res. 42: 4780–4790 (2008)
Farre MJ, Franch MI, Malato S, Ayllon JA, Peral J, Domenech X. Degradation of some biorecalcitrant pesticides by homogeneous and heterogeneous photocatalytic ozonation. Chemosphere 58: 1127–1133 (2005)
Hickling A. p. 329. In: Modern Aspects of Electrochemistry 6. ai]Bocklis JOM, Conway BE (eds). Plenum Press, NY, USA (1971)
Sengupta SK, Singh R Srivastava AK. A study on the origin of nonfaradaic behavior of anodic contact glow discharge electrolysis: The relationship between power dissipated in glow discharges and nonfaradaic yields. J. Electrochem. Soc. 145: 2209–2213 (1998)
Pu L, Gao J, Hu Y, Liang H, Xiao W Wang X. Oxidation degradation of aqueous carbofuran induced by low temperature plasma. Plasma Sci. Technol. 10: 348–351 (2008)
Chen F, Zeng L, Zhang Y, Liao X, Ge Y, Hu X Jiang L. Degradation behavior of methamidophos and chlorpyrifos in apple juice treated with pulsed electric fields. Food Chem. 112: 956–961 (2009)
Gao J, Wang X, Hu Z, Deng H, Hou J, Lu X Kang J. Plasma degradation of dyes in water with contact glow discharge electrolysis. Water Res. 37: 267–272 (2003)
Kang S-H, Choi W. Oxidative degradation of organic compounds using zero-valent iron in the presence of natural organic matter serving as an electron shuttle. Environ. Sci. Technol. 43: 878–883 (2009)
Bokare AD, Choi W. Chromate-induced activation of hydrogen peroxide for oxidative degradation of aqueous organic pollutants. Environ. Sci. Technol. 44: 7232–7237 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, SM., Min, Z.W., Mok, C. et al. Aqueous degradation of imidacloprid and fenothiocarb using contact glow discharge electrolysis: Degradation behavior and kinetics. Food Sci Biotechnol 22, 1773–1778 (2013). https://doi.org/10.1007/s10068-013-0279-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-013-0279-2