Abstract
Background
There are currently no validated criteria that entirely explain or predict response to methotrexate (MTX) treatment in rheumatoid arthritis (RA). We tried to identify the connection between three variants (RFC1 G80A (rs1051266), TYMS 2R/3R (rs34743033), and ATIC C347G (rs2372536)) in the folate pathway of MTX metabolism and the response to MTX monotherapy in a cohort of RA cases.
Methods
A prospective study on 100 RA patients on MTX monotherapy was performed. Disease activity was measured at the start of treatment and 6 months after treatment with MTX. The patients were then split into two groups: those who responded to the treatment and those who did not. The molecular genetic study for the RFC1 (G80A) variant was employed via the PCR-restriction fragment length polymorphism (PCR–RFLP) technique, the ATIC (C347G) variant was performed using TaqMan allelic discrimination real-time PCR, and the tandem repeat sequences of TYMS (2R/3R) were amplified by conventional PCR and detected by agarose gel electrophoresis.
Results
The genotype distribution of RFC-1 (G80A) showed significant variations among non-responders and responders in the recessive genetic model. A significant difference was found in TYMS (2R/3R) in the dominant and heterozygous genetic models. However, ATIC (C347G) genotype frequency did not exhibit substantial link with drug response in all genetic models. Furthermore, the genotype and allele rates of the analyzed variants did not show any significant association with adverse events in all genetic models.
Conclusion
The 80AA genotype of RFC-1 G80A and the 2R/3R or 3R/3R genotypes of TYMS 2R/3R are more vulnerable to the good consequences of MTX therapy.
Key Points |
• Current recommendations support the gold standard role of MTX as a first-line monotherapy for RA patients. However, up to 40% of RA patients do not respond or exhibit partial response to MTX. • Persistent disease activity due to treatment unresponsiveness will affect the long-term outcomes in RA patients. • We aimed, through molecular genetic study, to identify the connection between three variants in the folate pathway of MTX metabolism and the response to methotrexate monotherapy in a cohort of RA patients. |
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a female-predominant autoimmune inflammatory illness. Its prevalence varies significantly between countries. It accounts for 0.5 to 1% of the population. RA predominantly affects the synovial joint lining, leading to joint pain and swelling, which results in joint deformity, physical disability, and ultimately a lower quality of life [1]. The major treatment goal after the diagnosis is to monitor disease activity and slow the rate of joint destruction while also decreasing pain, stiffness, inflammation, and possible complications [2]. To achieve complete hindering of the disease’s progress and clinical remission, rheumatologists must monitor the progression of the disease on a frequent and accurate basis and alter therapeutic regimens as warranted. A change in the disease activity score of 28 joints (DAS28) is a standard way to assess the therapy response [3].
Disease-modifying anti-rheumatic drugs (DMARDs) have received a lot of attention during the last two decades because they can effectively stop disease progress, delay or prevent joint destruction, and substantially improve the long-term functional outcome [4].
Methotrexate (MTX) (4-amino-10-methyl folic acid) is a highly effective conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) that is widely employed in RA therapy, either alone or in combination with other DMARDs [5, 6]. It is a folic acid structural analogue with potent anti-inflammatory and anti-proliferative properties. Following its administration and absorption, MTX enters the cell through reduced folate carrier-1 (RFC1), which is encoded via the human solute carrier family 19, member 1 (SLC19A1), or RFC1 gene on chromosome 21. It was reported that genetic variants in the RFC1 gene influenced RFC1 function, affecting MTX transport [1]. Within the cell, MTX is transformed into methotrexate polyglutamates (MTX-PG), which direct the long-term suppression of the folate pathway and provide single-carbon moieties required for purine and pyrimidine biosynthesis [7]. They inhibit important intracellular enzymes within the folate pathway involving dihydrofolate reductase (DHFR), thymidylate synthetase (TS), and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) transformylase (ATIC) [8].
Thymidylate synthase is an enzyme that transforms deoxyuridylate into deoxythymidylate, which is needed for DNA synthesis and repair [9]. It is hindered by MTX-PG, thus participating in MTX anti-inflammatory and anti-proliferative properties [1]. Some variants in the TYMS gene, which encodes TS, look to be linked to MTX responsiveness. A genetic variant in the enhancer region (TSER) is TYMS-TSER-2R/3R, which comprises two or three 28-bp tandem repeats. The inclusion of a triple rather than a double 28-bp repeat has been demonstrated to enhance TYMS expression and might necessitate a larger MTX dosage to produce an effective therapeutic outcome; however, the potential implications of this variant on enzyme activity are still debated [10].
The ATIC enzyme changes AICAR into formyl-AICAR, which is then implicated in the de novo production of purine. Inhibition of ATIC by MTX leads to an intracellular piling up of AICAR, causing the emission of adenosine into the extracellular space. The adenosine released elevates cyclic adenosine monophosphate (cAMP), which prevents the release of pro-inflammatory cytokines that serve critical functions in the inflammatory process in RA [1].
Although MTX is the mainstay of RA treatment, toxicity remains a concern. A total of 30 to 45% of RA patients who take MTX have side effects, and 16% stop taking it because of side effects like hepatotoxicity, interstitial pneumonia, renal failure, pancytopenia, gastrointestinal dysfunction, and elevated susceptibility to infection [11].
The causes of individual non-responsiveness and the existence of adverse reactions are still hard to know. Genetic markers have recently received a lot of attention as potential indicators of MTX efficacy and toxicity. The goal of this research is to assess the relationship among RFC1 G80A (rs1051266), TYMS-2R/3R (rs34743033), and ATIC C347G (rs2372536) variants found in the MTX metabolic cascade and the outcome (response to/or toxicity of) methotrexate therapy in Egyptian patients with RA.
Subjects and methods
Study population
This study is a prospective cohort experiment employed from July 2022 to April 2023. It was carried out directly after the approval of the Ethical Committee of the Faculty of Medicine, Alexandria University (approval no. 0305722, IRB no. 00012098). Informed consent was received from all people involved in the study. A hundred early rheumatoid arthritis (symptom duration ≤ 2 years) or newly diagnosed cases were consecutively gathered from the rheumatology outpatient clinic and inpatient unit in the Rheumatology Unit, Alexandria Main University Hospital. The detection of RA was based on the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA [12]. The inclusion criteria included DMARD-naïve patients with active disease (DAS28-ESR > 3.2) [13], and only cases treated with MTX as csDMARD monotherapy were selected. Other additional medications included oral prednisolone in a dose of ≤ 10 mg/day or its equivalent (with a tapering regimen), non-steroidal anti-inflammatory drugs (NSAIDs), and folic acid, which were allowed to be taken during the study period. The exclusion criteria included patients with MTX intolerance, patients receiving csDMARDs other than methotrexate, as well as biologic (bDMARD) and targeted synthetic DMARDs (tsDMARDs).
The patients were assessed at baseline, after 3 months, and after 6 months post therapy with methotrexate. Starting doses ranged between 12.5 and 25 mg/week, regardless of the route of administration. During follow-up visits, patients who developed any of the adverse effects of MTX have been asked to reduce the dose of MTX to the lowest tolerated dose, which was 7.5 mg/week in our cohort. Then, they were categorized into a responders’ group (41 patients) and a group of non-responders (59 patients) according to the reassessment at the follow-up visit after 6 months. The non-responders are those who remained on MTX for 6 months (the study peroid) but did not exhibit enough improvement in line with EULAR response criteria (DAS28 improvement of ≤ 0.6 from baseline score), whereas the responders are those who showed improvement in their DAS28 ≥ 0.6 from baseline assessment [14]. Patients who had discontinued the MTX before completing 6 months due to MTX intolerance, adverse events, inefficacy, or required bDMARD or tsDMARDs have been excluded from the study.
Furthermore, the subjects were divided into cases with adverse events (n = 52) and cases without adverse events (n = 48) according to the development of any of the known MTX adverse effects.
Methods
All of the following were accomplished for all of the patients who were enrolled in the study: an entire history taking with stress on the onset of the disease; a history of smoking, periodontitis, and joint symptoms (pain, tenderness, swelling, morning stiffness, and difficulty in movement); and a physical examination with stress on the number of tender and/or swollen joints, limitations of motion, deformities, rheumatoid nodules, extra-articular manifestations, and the presence, if any, of a low-grade fever and other constitutional manifestations. Disease activity estimations in the form of DAS28-ESR (scale 0–9), functional assessment by using the health assessment questionnaire (HAQ) (score range 0–3), and imaging tests for joints in the form of an X-ray or ultrasound were done on any patient if required.
Laboratory investigations
Rheumatoid factor (RF) and anti-cyclic citrullinated protein antibody (Anti-CCP) have been requested for all participants. Routine laboratory tests including complete blood count (CBC), serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, creatinine, estimated glomerular filtration rate (eGFR), and inflammatory markers involving erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were done for all cases at baseline (time of recruitment), after 3 months (follow-up visit), and after 6 months of treatment with MTX (end of study).
Molecular genetic studies
DNA extraction
Whole venous blood specimens were gathered on Vacutainer ethylenediaminetetraacetic acid (EDTA) blood collection tubes from all patients. Genomic DNA extraction by column-based extraction kits (QIAamp DNA Blood Mini Kit, Catalogue number: 51104) was performed depending on the manufacturer’s manuals. The purity and concentration of DNA were identified by the Thermo Scientific NanoDrop™ 1000A Spectrophotometer at 260 and 280 nm.
RFC1 genotyping
The genotyping of RFC1 G80A was employed via the polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) assay. It was employed in a 25 µL reaction volume, utilizing 10 pmol forward primer (5-AGTGTCACCTTCGTCCCCTC-3′), 10 pmol reverse primer (5-CTCCCGCGTGAAGTTCTT-3′), and 12.5 µL COSMO “Hot Start” PCR RED Master Mix (Willowfort, UK). The PCR conditions were performed as previously described [15]. It started with a 5-min denaturation at 95 °C, then, 35 cycles of denaturation for 15 s at 95 °C, annealing at 60 °C for 1 min, extension for 30 s at 72 °C, and a final extension at 72 °C for 7 min.
A total of 25 µL of the PCR products (230 bp) were broken down with 1 µL of the Fast Digest HhaI enzyme (FD1854, Thermo Fisher Scientific, USA) for 5 min at 37 °C as described by the manufacturer. The digested PCR product was run on 3% agarose gel electrophoresis and stained with ethidium bromide. Individuals with the 80GG genotype had three fragments (125, 68, and 37 bp), those with the 80GA genotype had four fragments (162, 125, 68, and 37 bp), and those with the 80AA genotype had two fragments (162 and 68 bp) (Fig. 1).
Agarose gel electrophoresis (3%) of the PCR products digested with the Fast Digest HhaI restriction enzyme. Lanes 5: 50 bp DNA Ladder (50–1000 bp); Lanes 1, 2, and 3: 80 GA heterozygote alleles (bands at 162, 125, 68, and 37 bp); Lanes 4, 7, and 8: 80 AA homozygote alleles (bands at 162 bp, and 68 bp); Lane 6: 80 GG homozygote alleles (band at 125, 68, and 37 bp)
TYMS genotyping
Standard PCR was used to amplify the TYMS genomic area, and the tandem repeat sequences in the 5′-end of the TYMS regulatory region [TYMS 2R/3R (rs34743033)] were detected. It was performed in a 25-μL reaction volume, via 10 pmol forward primer (5′‐GTGGCTCCTGCGTTTCCCCC‐3′), 10 pmol reverse primer (5′-GCTCCGAGCCGGCCACAGGCATGGCGCGG-3), and 12.5 µL COSMO “Hot Start” PCR RED Master Mix (Willowfort, UK). The PCR condition was performed as described previously, with modifications [16]. It comprised a 5-min initial denaturation at 94 °C, then proceeded to 35 cycles with denaturation for 1 min at 94 °C, annealing at 60 °C for 30 s, extension for 30 s at 72 °C followed by a final extension at 72 °C, for 10 min.
The PCR product was directly run on 3% agarose gel electrophoresis and stained with ethidium bromide. In the existence of homozygotes of the double repeat (TYMS 2R2R), a 220-bp fragment was created, while heterozygotes (TYMS 2R3R) produced two fragments of 220 and 248 bp. In the existence of homozygous TYMS triple repeat (TYMS 3R3R), a fragment with 248 bp was generated (Fig. 2).
Agarose gel electrophoresis (3%) of PCR products of the amplified tandem repeat sequences in the 5′-terminal of the TYMS regulatory region. Lane 5: 50 bp DNA Ladder (50–1000 bp); Lanes 1, 4, and 8: heterozygotes (TYMS 2R3R) produced bands at 220 bp and 248 bp; Lanes 2 and 3: homozygotes of the double repeats (TYMS 2R2R), produced bands at 220 bp; Lanes 6 and 7: homozygotes of the triple repeats (TYMS 3R3R) produced bands at 248 bp
ATIC genotyping
The ATIC (C347G) genotyping was done with TaqMan allelic real-time PCR and fluorogenic binding probes. A ready-made TaqMan SNP Genotyping Assay (C__16218146_10; catalogue number: 4351379) from Thermo Fisher Scientific was employed. The reaction volume was 20 µL for each sample. It was made up of 10 µL of TaqMan Genotyping Master Mix (catalogue number: 4371353; Thermo Fisher Scientific), 3.0 µL of extracted DNA, 1.0 µL of 20X SNP assay mixture (primers and probes), and 6.0 µL of nuclease-free water. The following thermal cycling circumstances were implemented: 10 min at 95 °C for enzyme activation, 40 cycles of 15 s at 95 °C for denaturation, and 1 min at 60 °C for annealing and extension. TaqMan Genotyper Software (Applied Biosystems) was used to analyze the data for genotyping, and allelic discrimination plots were constructed for each run to visualize the genotypes of the complete cohort.
Statistical analysis
The sample size was calculated via NCSS 2004 and PASS 2000 software. It recorded 90% power to identify an effect size (W) of 0.4 in the treatment response utilizing a 2-degree-of-freedom Chi-square test with a significance level (alpha) of 0.05 considering a 10% loss follow-up rate. Data was entered into the computer and processed with the IBM SPSS software program, version 20.0. IBM Corporation, Armonk, New York. To compare the two categorical groups, the chi-square test was utilized. When greater than 20% of the cells had an anticipated enumeration of less than 5, the Fisher Exact or Monte Carlo correction test was applied. The Kolmogorov–Smirnov test was implemented to determine the normality of continuous data. For normally distributed quantitative variables, the student t test and paired t test were applied. For not-normally distributed quantitative parameters, the Mann–Whitney test and Wilcoxon signed rank tests were applied. By comparing expected and observed genotype frequencies, the agreement with the Hardy–Weinberg equilibrium (HWE) was determined. All genotype frequencies were in HWE, except for the frequency of the RFC-1 genotype, which deviated from HWE. This is due to the overrepresentation of the heterozygous genotype frequency. Under homozygous, heterozygous, dominant, and recessive genetic models, we calculated genotype-specific odds ratios (ORs). People who were homozygous for the common allele served as referents for homozygous, heterozygous, and dominant models. We utilized those with one or two copies of the common allele as the reference group for the recessive model. The acquired findings were declared significant at the 5% level. In addition, univariate logistic regression analysis was implemented to determine the relationship between methotrexate non-response and the individual gene polymorphisms, age, gender, and other clinical factors.
Results
Patients’ clinical characteristics
A total of 100 cases of rheumatoid arthritis were on MTX monotherapy. According to the EULAR response criteria, they were subdivided into 41 patients as responders, and their mean age was 39.2 ± 7.9 years, and 59 patients as non-responders, and their mean age was 39.0 ± 8.7 years. Thirty-two females were present in the responders’ group, while 46 females were found in the non-responders group. The median disease duration at the time of recruitment in the non-responders group was 10.0 (3.0–24.0) month, and in the responders group was 9.0 (3.0–24.0) month, with no significant difference between the two groups (p = 0.251). By analyzing the relation between the patients’ characteristics and the clinical response to methotrexate, no significant differences were observed between the two groups as regards smoking (p = 0.646), body mass index (0.524), as well as comorbidity such as diabetes mellitus (p = 0.440) and hypertension (p = 0.13).
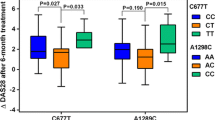
Baseline activity markers and disease activity were measured in all patients using the ESR, CRP, DAS 28-ESR, and HAQ score, and then they were re-measured 3 (not shown) and 6 months after MTX therapy MTX. The results showed no statistically significant difference between the two groups at the baseline assessment in ESR, CRP, DAS28-ESR (p = 0.067, 0.077, 0.992, respectively), whereas baseline HAQ score showed significant higher value in responder than in non-responder group (p = 0.016). The baseline and follow-up activity markers and disease activity score of the two studied groups are presented in Table 1
Routine laboratory investigations
The comparison among the non-responders’ and responders’ groups regarding routine laboratory investigations including hemoglobin, white blood count, platelets, AST, creatinine, and eGFR revealed no significant differences between the two groups with p = 0.102, p = 0.526, p = 0.329, p = 0.131, p = 0.902, and p = 0.984, respectively. However, there was significant difference in serum urea and ALT level with p ≤ 0.001.
Serology markers
Of the MTX responders, 87.8% were seropositive for RF, whereas 89.8% were positive in the non-responders’ group. The anti-CCP was positive in 85.4% of the responders’ group, while it was 91.5% positive in the non-responders’ group (Table 2).
Lines of treatment
The median dose of MTX given to both groups was 25 mg/week. GIT upset (36.6%) was the most frequent adverse event in the responders’ group, while excessive hair fall (32.2%) was the most frequent in the non-responders’ group. The details of the therapy given to the studied groups are represented in Table 2.
Genotyping and allele frequency
In the non-responders’ group, the allele frequencies of RFC-1 80G (rs1051266) and TYMS 2R (rs34743033) were 45.8% and 39.8%, respectively, while the frequency of the ATIC G (rs2372536) allele was 32.2%. Non-statistical significance was discovered among non-responders’ and responders’ groups in the allele models among all the studied variants. The allele and genotype rates of the analyzed variants among non-responders and responders are presented in Table 3.
The genotype distribution of RFC-1 G80A (rs1051266) showed significant differences among the non-responders and responders in the recessive, p = 0.009, OR = 0.272 (0.102–0.724), genetic model. Also, a significant difference was found in TYMS 2R/3R (rs34743033) among the dominant model, p = 0.022, OR = 0.165 (0.035–0.771), and the heterozygous genetic model, p = 0.013, OR = 0.129 (0.026–0.644). On the other hand, ATIC C347G (rs2372536) genotype frequency did not exhibit any significant link with the drug response in all the genetic models. A forest plot showing the link between the studied genetic parameters and the MTX response is represented in Fig. 3A.
A univariate regression was implemented to figure out the role of the various clinical factors in predicting the MTX outcome. Except for TYMS (rs34743033) (2R/2R) p = 0.022, OR = 6.067 (1.297–28.366), and RFC-1 (rs1051266) (GA + GG) p = 0.009, OR = 3.678 (1.381–9.794), none of the clinical variables retained significance in the univariate analysis. The multivariate regression analysis confirmed their role as risk factors for non-response to methotrexate (Table 4).
Furthermore, the genotype and allele rates of the analyzed variants did not show any significant association with the adverse reactions in all the genetic models, as shown in Table 5. A forest plot showing the relationship between the studied genetic parameters and the MTX toxicity is represented in Fig. 3B.
Discussion
Despite the patients’ compliance with treatment in the current research, only 41% of them exhibited a good response to MTX monotherapy at a median concentration of 25 mg/week, while 59% showed a poor response at the same median dose. In an observational study by Sergeant et al. [17], 43% of their cohort were classified as non-responders. Their baseline determinants of non-response in a multivariable logistic regression model were lower disease activity, negative RF, and a higher HAQ score. In this study, none of the different clinical elements revealed significance in the univariate regression analysis, and no clinical criteria could adequately explain or predict therapy response. So consideration of genetic factors affecting therapy response was more pertinent.
According to the current research, variations in MTX effectiveness and toxicity may be associated with MTX pathway dysregulation. Because multiple enzymes are involved in MTX metabolism, changes in enzyme accessibility and functioning may have a direct impact on MTX therapy [18].
Using the recessive genetic model, there was a significant difference in the RFC1 G80A (rs1051266) genotype distribution between non-responders and responders. This clarified that the 80 AA genotype of RFC1 G80A was linked to a better response to MTX treatment. Multivariate regression analysis confirmed this result. It showed that RFC1 (GA + GG) increased the risk of not responding to MTX by 3.838-fold.
RFC1, which has a high affinity for reduced folates and different hydrophilic antifolates like MTX, controls the active movement of MTX into the gastrointestinal system. It has been proposed that the RFC1 G80A variation in exon 2 is linked to the effectiveness of MTX. It causes the replacement of histidine for arginine at amino acid position codon 27 in the first transmembrane domain of the RFC1 protein [19]. A previous study done in silico showed that this variant changed the topology of RFC1 at the S1, S2, S4, S8, S9, and S10 transmembrane domains. The S2 domain is shorter in the mutant protein than in the wild protein by three amino acid residues, while the difference in other domains corresponds to one amino acid residue. This alters how tetrahydrofolate (THF), 5-methyl-THF, and MTX are transported across the membrane [20]. It was previously shown that RFC1 mRNA, which is affected by gene variants, has been found to affect the beneficial effects of MTX in RA [21].
In agreement with the current result, Baslund et al. [22] found that individuals with the A variant (80AA and 80GA) had higher levels of MTX uptake in antigen-stimulated CD4 + T cells and B cells than those exhibiting the 80GG variant, which may have contributed to an increase in MTX influx. Both cells are crucial in the pathogenesis of RA. Similarly, a study on Indians demonstrated that those with the RFC1 80 AA genotype responded to MTX better than people with the RFC1 80 GG genotype [23]. Furthermore, Dervieux et al. reported that RFC1 80AA homozygosity was linked to 3.4-fold greater amounts of MTX-PG3-5 and/or MTX-PG5 in red blood cells [24]. Also, Naushad et al. [25] showed that the RFC1 80A-allele increased the usefulness of MTX therapy by 1.53-fold in their meta-analysis, which included 18 studies and was representative of 3592 RA individuals, and the 80AA-genotype raised the impact by 1.85-fold with an increased MTX dose equivalent to 15 mg/week, which is comparable to the average dose in the current study. Another study demonstrated that cases with the RFC1 80AA genotype had a 3.32-fold greater chance of remission of RA manifestations than those with the GG genotype [26].
On the other hand, Yamamoto’s study [8], which went against the current findings, showed that there was no link between the intracellular MTXPG3-5/1–2 ratios and the RFC-1 G80A polymorphism. Earlier studies also found no differences between the RFC-1 gene polymorphisms and the effectiveness of MTX therapy in Chinese and European cohorts of RA patients [7, 27]. Although the outcomes of the present study illustrated that RFC-1 G80A may be an important pharmacogenetic indicator of MTX treatment, more studies in different populations with a larger sample size are highly recommended.
TYMS is an enzyme implemented in DNA synthesis and repair. Its inhibition by MTX-PG contributes to MTX anti-proliferative and anti-inflammatory properties. The genetic variation in the TYMS gene, which involves a tandem repeat of a 28-base pair sequence in the promoter region, resulting in a 2R or 3R allele, may affect the transcriptional activity of the gene [1]. However, its potential consequences on enzyme function are still a matter of controversy, prompting a search approach to better understand the influence of this variation on pharmacogenetics. In the current study, a significant difference was found among the non-responders and responders in the dominant and codominant genetic models of TYMS 2R/3R, indicating that the presence of 2R/3R or 3R/3R genotypes makes them more vulnerable to a good response. The multivariate regression analysis confirmed the role of TYMS (rs34743033) (2R/2R) by 6.39-fold as a risk factor for non-response to MTX.
Subjects with the TYMS 3R repeat sequence exhibited higher translational efficiency than those with the TYMS 2R repeat sequence. The increased expression of the 3R allele can boost the conversion of dUMP to dTMP, reducing the amount of uracil that could get integrated into DNA and cause double strand breaks [28].
In agreement with the current result, James et al. [29] revealed that patients with the TYMS 3R3R genotype reacted more positively to therapy in 98 early RA patients. On the contrary, Lima et al. [30] stated that the TYMS 3R allele favored non-response to MTX medication in Portuguese Caucasian RA people. Although there were significant univariate analysis outcomes in their study, the multivariate test was not capable of supporting this result. Also, Muralidharan et al. [31] found that the 3R allele was greater in non-responders than in persons having remission and concluded that the TYMS 3R allele could provoke non-response to MTX in South Indian Tamil ethnicity. Other study [27] assumed that the TYMS 28-bp tandem repeat variation did not have any function in anticipating the MTX therapy outcome. Despite the promise of our findings, important study constraints, such as the relatively small population size and the presence of additional TYMS variations that may influence TS expression or activity and necessitate further research, may help explain these disparities in the results.
Besides the TYMS, MTX-PGs target the ATIC enzyme, which prompts the last two steps of de novo purine production, and raise the intracellular concentration of AICAR, which contributes to the activation of the adenosine signaling pathway. Strong anti-inflammatory functions are produced by the release of adenosine, which reduces neutrophil adhesion and suppresses the function of natural killer cells, monocytes, macrophages, and T lymphocytes [32]. The ATIC C347G (rs2372536) variant on exon 5 is an ATIC variation that changes the amino acid threonine to the amino acid serine at position 116 of the gene. The current study failed to find strong evidence linking the ATIC C347G variation to medication response in any of the genetic models investigated. This finding was previously reported in other research. Sha et al. [18] evaluated the ATIC C347G polymorphism in 647 RA patients and found that this variation had no effect on MTX efficacy based on both the chi-square test and binary logistic regression. The stratification of RA patients by self-reported ancestry, however, showed that Malay RA patients with minor allele G of ATIC responded better to MTX therapy than Chinese and Indian RA patients. Additionally, Sharma and Muralidharan [33, 34] found no correlation between MTX responsiveness in Indian people and ATIC C347G in their investigations.
However, a link between the ATIC 347 GG + GC genotype and non-response to MTX treatment was discovered in the Caucasian population by a meta-analysis based on five experiments with 1056 RA people. Another meta-analysis presented a significant link among the frequency of the ATIC C347G allele and the state of the MTX response in both dominant and codominant models [32, 35]. A cross-sectional investigation of 108 RA cases by Dervieux et al. [15] found that individuals with a homozygous GG of ATIC C347G could possess a greater percentage of excellent reactions to MTX than those with a CC or CG genotype. Kurzawski et al. [36] came to identical findings in RA patients from a Caucasian population.
The differences across the studies might be attributed to the varying selection criteria used for the studied individuals, their varied ethnicities, geographic locations, concurrent environmental risk factors, and interactions between genes and their environment.
MTX medication has been discontinued in around 30% of patients owing to adverse effects, according to estimates. In the current investigation, the genotypes and allele frequencies of the selected variations did not show any significant associations with the deleterious effects across all genetic models.
According to Wang et al.’s findings [37], RFC1 G80A gene polymorphisms were not connected to MTX toxicity in Chinese Han individuals, which lines up with our results. Muralidharan et al. reported the same results in the Indian population [31] and regardless of ethnicity, the meta-analysis of Huang et al. [38] which comprised seven studies and came to the same conclusions.
The relationships between the chosen variants and MTX toxicity, however, varied among researchers. People with the RFC1 80AA genotype were shown to possess a greater prevalence of MTX-related hepatotoxicity and alopecia, while the TYMS 3R3R genotype was linked to a greater danger of bone marrow toxicity. Instead, Chaabane et al. [39] demonstrated that the TYMS 2R/3R repeat variant had a protective impact as MTX toxicity developed. Furthermore, Grabar et al. [40] recorded that ATIC 347G allele carriers possess a 2.5-fold increased risk of MTX-induced toxicity compared to non-carriers.
The results’ variability may be caused by changes in clinical characteristics, discrepancies in how treatment outcomes are measured, inter-ethnic differences, and an insufficient sample size. To better recognize the function of these and other genetic variations in methotrexate response and to identify patient subgroups who are more likely to experience methotrexate failure and may benefit from alternative therapies, additional research in a larger, prospectively gathered cohort with well-defined outcomes and clinical measures will be necessary. These results need to be verified by other research, and if they are, they may emphasize the need for tailored treatment for RA patients.
Conclusion
In our RA cohort, the 80AA genotype of RFC-1 G80A and the 2R/3R or 3R/3R genotypes of TYMS 2R/3R were more vulnerable to a good response to MTX treatment. Furthermore, no clinical variables could be linked with a poor response to MTX treatment.
Data availability
This paper includes all data created or analyzed during this investigation.
Abbreviations
- ACR :
-
American College of Rheumatology
- ALT :
-
Alanine aminotransferase
- Anti-CCP :
-
Anti-cyclic citrullinated protein antibody
- AST :
-
Aspartate aminotransferase
- BMI :
-
Body mass index
- CBC :
-
Complete blood count
- CRP :
-
C-reactive protein
- DAS :
-
Disease activity score
- EDTA :
-
Ethylenediaminetetraacetic acid
- eGFR :
-
Estimated glomerular filtration rate
- EULAR :
-
European league against rheumatism
- ESR :
-
Erythrocyte sedimentation rate
- HAQ :
-
Health assessment questionnaire
- MTX :
-
Methotrexate
- NSAIDs :
-
Nonsteroidal anti-inflammatory drugs
- PCR–RFLP :
-
Polymerase chain reaction-restriction fragment length polymorphism
- RA :
-
Rheumatoid arthritis
- RF :
-
Rheumatoid factor
References
Szostak B, Machaj F, Rosik J, Pawlik A (2020) Using pharmacogenetics to predict methotrexate response in rheumatoid arthritis patients. Expert Opin Drug Metab Toxicol 16(7):617–626
Bullock J, Rizvi SA, Saleh AM, Ahmed SS, Do DP, Ansari RA et al (2019) Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract 27(6):501–507
Hensor EM, McKeigue P, Ling SF, Colombo M, Barrett JH, Nam JL et al (2019) Validity of a two-component imaging-derived disease activity score for improved assessment of synovitis in early rheumatoid arthritis. Rheumatology 58(8):1400–1409
Smolen JS, Landewé RB, Bijlsma JW, Burmester GR, Dougados M, Kerschbaumer A et al (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79(6):685–699
Sanmartí R, García-Rodríguez S, Álvaro-Gracia JM, Andreu JL, Balsa A, Cáliz R et al (2015) 2014 update of the Consensus Statement of the Spanish Society of Rheumatology on the use of biological therapies in rheumatoid arthritis. Reumatología Clínica (English Edition) 11(5):279–294
Brown PM, Pratt AG, Isaacs JD (2016) Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol 12(12):731–742
Lv S, Fan H, Yang H, Huang J, Li J, Shu X et al (2019) Membrane-spanning protein genetic polymorphisms related to methotrexate therapeutic outcomes in a Chinese rheumatoid arthritis population. J Clin Pharmacol 59(11):1471–1476
Yamamoto T, Shikano K, Nanki T, Kawai S (2016) Folylpolyglutamate synthase is a major determinant of intracellular methotrexate polyglutamates in patients with rheumatoid arthritis. Sci Rep 6(1):35615
Lan X, Field MS, Stover PJ (2018) Cell cycle regulation of folate-mediated one-carbon metabolism. Wiley Interdiscip Rev Syst Biol Med 10(6):e1426
Conti V, Corbi G, Costantino M, De Bellis E, Manzo V, Sellitto C et al (2020) Biomarkers to personalize the treatment of rheumatoid arthritis: focus on autoantibodies and pharmacogenetics. Biomolecules 10(12):1672
Lima A, Bernardes M, Sousa H, Azevedo R, Costa L, Ventura F et al (2014) SLC19A1 80G allele as a biomarker of methotrexate-related gastrointestinal toxicity in Portuguese rheumatoid arthritis patients. Pharmacogenomics 15(6):807–820
Funovits J, Aletaha D, Bykerk V, Combe B, Dougados M, Emery P et al (2010) The 2010 American College of Rheumatology/European League against rheumatism classification criteria for rheumatoid arthritis: methodological report phase I. Ann Rheum Dis 69(9):1589–1595
Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS (2005) Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 52(9):2625–2636
Fransen J, Van Riel PL (2009) The disease activity score and the EULAR response criteria. Rheum Dis Clin 35(4):745–757
Dervieux T, Furst D, Lein DO, Capps R, Smith K, Walsh M et al (2004) Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum 50(9):2766–2774
Rahimi Z, Zarini MB, Rahimi Z, Shakiba E, Vaisi-Raygani A, Moradi MT et al (2021) Variants of genes involved in metabolism of folate among patients with breast cancer: association of tyms 3R allele with susceptibility to breast cancer and metastasis. Iran J Pathol 16(1):62
Sergeant JC, Hyrich KL, Anderson J, Kopec-Harding K, Hope HF, Symmons DP et al (2018) Prediction of primary non-response to methotrexate therapy using demographic, clinical and psychosocial variables: results from the UK Rheumatoid Arthritis Medication Study (RAMS). Arthritis Res Ther 20:1–1
Sha HX, Veerapen K, Chow SK, Gun SC, Lau IS, Lim RL et al (2022) Genetic variations in methotrexate metabolic pathway genes influence methotrexate responses in rheumatoid arthritis patients in Malaysia. Sci Rep 12(1):11844
Abdallah HY, Ibrahim ME, Abd El-Fadeal NM, Ali DA, Elsehrawy GG, Badr RE et al (2022) Pharmacogenomics of methotrexate pathway in rheumatoid arthritis patients: approach toward personalized medicine. Diagnostics 12(7):1560
Naushad SM, Devi AR, Hussain T, Alrokayan SA, Ramaiah MJ, Kutala VK (2019) In silico analysis of the structural and functional implications of SLC19A1 R27H polymorphism. J Genet 98:1–8
Tazoe Y, Hayashi H, Tsuboi S, Shioura T, Matsuyama T, Yamada H et al (2015) Reduced folate carrier 1 gene expression levels are correlated with methotrexate efficacy in Japanese patients with rheumatoid arthritis. Drug Metab Pharmacokinet 30(3):227–230
Baslund B, Gregers J, Nielsen CH (2008) Reduced folate carrier polymorphism determines methotrexate uptake by B cells and CD4+ T cells. Rheumatology 47(4):451–453
Ghodke-Puranik Y, Puranik AS, Shintre P, Joshi K, Patwardhan B, Lamba J et al (2015) Folate metabolic pathway single nucleotide polymorphisms: a predictive pharmacogenetic marker of methotrexate response in Indian (Asian) patients with rheumatoid arthritis. Pharmacogenomics 16(18):2019–2034
Dervieux T, Kremer J, Lein DO, Capps R, Barham R, Meyer G et al (2004) Contribution of common polymorphisms in reduced folate carrier and γ-glutamylhydrolase to methotrexate polyglutamate levels in patients with rheumatoid arthritis. Pharmacogenet Genomics 14(11):733–739
Naushad SM, Alrokayan SA, Almajhdi FN, Hussain T (2021) Influence of RFC1 c. 80A> G polymorphism on methotrexate-mediated toxicity and therapeutic efficacy in rheumatoid arthritis: a meta-analysis. Ann Pharmacother 55(12):1429–38
Drozdzik M, Rudas T, Pawlik A, Gornik W, Kurzawski M, Herczynska M (2007) Reduced folate carrier-1 80G> A polymorphism affects methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenomics J 7(6):404–407
Owen SA, Hider SL, Martin P, Bruce IN, Barton A, Thomson W (2013) Genetic polymorphisms in key methotrexate pathway genes are associated with response to treatment in rheumatoid arthritis patients. Pharmacogenomics J 13(3):227–234
Lima A, Azevedo R, Sousa H, Seabra V, Medeiros R (2013) Current approaches for TYMS polymorphisms and their importance in molecular epidemiology and pharmacogenetics. Pharmacogenomics 14(11):1337–1351
James HM, Gillis D, Hissaria P, Lester S, Somogyi AA, Cleland LG et al (2008) Common polymorphisms in the folate pathway predict efficacy of combination regimens containing methotrexate and sulfasalazine in early rheumatoid arthritis. J Rheumatol 35(4):562–571
Lima A, Seabra V, Bernardes M, Azevedo R, Sousa H, Medeiros R (2014) Role of key TYMS polymorphisms on methotrexate therapeutic outcome in Portuguese rheumatoid arthritis patients. PLoS One 9(10):e108165
Muralidharan N, Misra DP, Jain VK, Negi VS (2017) Effect of thymidylate synthase (TYMS) gene polymorphisms with methotrexate treatment outcome in south Indian Tamil patients with rheumatoid arthritis. Clin Rheumatol 36:1253–1259
Ghodke Y, Chopra A, Joshi K, Patwardhan B (2008) Are thymidylate synthase and methylene tetrahydrofolate reductase genes linked with methotrexate response (efficacy, toxicity) in Indian (Asian) rheumatoid arthritis patients? Clin Rheumatol 27:787–789
Sharma S, Das M, Kumar A, Marwaha V, Shankar S, Singh P et al (2009) Purine biosynthetic pathway genes and methotrexate response in rheumatoid arthritis patients among north Indians. Pharmacogenet Genomics 19(10):823–828
Muralidharan N, Mariaselvam CM, Jain VK, Gulati R, Negi VS (2016) ATIC 347C> G gene polymorphism may be associated with methotrexate-induced adverse events in south Indian Tamil rheumatoid arthritis. Pharmacogenomics 17(3):241–248
Qiu Q, Huang J, Shu X, Fan H, Zhou Y, Xiao C (2017) Polymorphisms and pharmacogenomics for the clinical efficacy of methotrexate in patients with rheumatoid arthritis: a systematic review and meta-analysis. Sci Rep 7(1):44015
Kurzawski M, Malinowski D, Szarmach N, Nowak A, Goryniak A, Pawlik A et al (2016) ATIC missense variant affects response to methotrexate treatment in rheumatoid arthritis patients. Pharmacogenomics 17(18):1971–1978
Wang S, Zuo S, Liu Z, Ji X, Yao Z, Wang X (2020) Association of MTHFR and RFC1 gene polymorphisms with methotrexate efficacy and toxicity in Chinese Han patients with rheumatoid arthritis. J Int Med Res 48(2):0300060519879588
Huang J, Fan H, Qiu Q, Liu K, Lv S, Li J et al (2020) Are gene polymorphisms related to adverse events of methotrexate in patients with rheumatoid arthritis? A retrospective cohort study based on an updated meta-analysis. Ther Adv Chronic Dis 11:2040622320916026
Chaabane S, Marzouk S, Akrout R, Ben Hamad M, Achour Y, Rebai A et al (2016) Genetic determinants of methotrexate toxicity in Tunisian patients with rheumatoid arthritis: a study of polymorphisms involved in the MTX metabolic pathway. Eur J Drug Metab Pharmacokinet 41:385–393
Grabar PB, Rojko S, Logar D, Dolžan V (2010) Genetic determinants of methotrexate treatment in rheumatoid arthritis patients: a study of polymorphisms in the adenosine pathway. Ann Rheum Dis 69(5):931–932
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AMN identified the study topic, conducted the research design and literature review, carried out molecular genetic investigations and laboratory experiments, analyzed and assessed the findings, and made a major contribution to the paper’s manuscript and reference collection. AA and RE participated in the research idea, patient clinical assessment, interpretation and analysis of the clinical data, participated in the writing of the manuscript, results and data interpretation, and paper revision. FD, HMN, and YNK participated in the research idea, writing of the manuscript and data interpretation, performed the molecular genetic studies and the laboratory work. All authors contributed to the writing and critical revision of the work. The final version to be published was accepted by all writers. The document was written without the involvement of a medical author.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The investigation was authorized by the Ethical Committee of the Faculty of Medicine, Alexandria University (Approval no. 0305722, IRB no. 00012098), and the experiment was employed in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All participants gave their informed consent.
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nomair, A.M., Abdelati, A., Dwedar, F.I. et al. The impact of folate pathway variants on the outcome of methotrexate therapy in rheumatoid arthritis patients. Clin Rheumatol 43, 971–983 (2024). https://doi.org/10.1007/s10067-024-06892-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-024-06892-w