Abstract
The objective of this study is to examine the proportion of the total treatment effect that is attributable to contextual effects in randomised controlled trials (RCTs) of treatments for fibromyalgia. A systematic literature search was undertaken in Medline, Web of Science, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Allied and Complementary Medicine in September 2015. The proportion of contextual effect (PCE) was calculated by dividing the improvement in the placebo arm by the improvement in the treatment arm. The measure was log-transformed for each trial and the random effects model was used to pool data. The primary outcome was pain. Secondary outcomes were fibromyalgia impact questionnaire (FIQ) total and fatigue. Heterogeneity was quantified using I 2. Publication bias was assessed using a funnel plot and Egger’s test. Subgroup analysis was undertaken to explore heterogeneity and potential determinants of the PCE. Fifty-one eligible trials (9599 participants) were identified. The PCE was 0.60 (95% CI 0·56 to 0·64) for pain, 0·57 (95% CI 0·53 to 0·61) for FIQ total, and 0·63 (95% CI 0·59 to 0·68) for fatigue. The I 2 was 99.4% for pain, 99.2% for FIQ total, and 97.6% for fatigue. More than half of the treatment effect in fibromyalgia RCTs results from non-specific contextual factors. This suggests that optimising contextual care may enhance treatment effects and improve outcomes. Reporting the total treatment effect and the proportion of contextual effect in trials may help to better translate research evidence into practice.
Similar content being viewed by others
Introduction
Fibromyalgia is a chronic painful and distressing condition with an estimated worldwide prevalence of 2.7% [1]. Recognised risk factors include female sex, increasing age, poor socioeconomic status, low education, and residence in rural areas [1]. The main symptoms are multiple regional musculoskeletal pain, stiffness, non-restorative sleep, fatigue, distress, and cognitive difficulties. Demonstrable abnormalities include widespread hyperalgesia (reduced pain threshold), allodynia, and reduced delta sleep [2]. Currently, there is no cure and the aim of treatment is to relieve symptoms and improve quality of life. An individualised multidisciplinary approach combining patient education, non-pharmacological and pharmacological treatments is recommended [3, 4].
Fibromyalgia treatments have been extensively evaluated in randomised controlled trials (RCTs). A meta-analysis of eight interventions (including tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin noradrenaline reuptake inhibitors (SNRIs), the gamma-amino butyric acid analogue pregabalin, aerobic exercise, balneotherapy, cognitive behavioural therapy (CBT), and multicomponent therapy) found that when lower quality studies are excluded the effect for pharmacological treatment becomes small and there is little evidence for effect from non-pharmacological treatments [5]. When studies with less than 100 participants in each arm were excluded the difference between treatments and placebo was judged to be too small to be clinically relevant.
The main focus in current reporting of RCTs is on the separation of the treatment group from the placebo and a treatment is deemed effective only if it is significantly better than placebo. That is, the judgement for treatment efficacy is based solely on the specific treatment effect. However, the benefit that a patient experiences derives not only from the specific effects of the treatment but also from the non-specific effects of the context in which the treatment is delivered [6]. This creates an ‘efficacy paradox’ when a patient in a clinical setting experiences significant benefit from a treatment that has been deemed ineffective in an RCT because it did not separate sufficiently from placebo [6]. This paradox is common with fibromyalgia, a condition that associates with a significant placebo effect in RCTs [7]. Therefore, to better reflect patient-centred experience, instead of evaluating a treatment solely on the difference between treatment and placebo (specific effect), we propose to additionally present the total treatment effect and the proportion attributed to contextual effects (PCE). The concept of PCE has been previously evaluated for the treatment of osteoarthritis and depression [6, 8]. Both studies revealed that the majority of the total treatment effects were contextual (exhibited in the placebo arm). The aim of this study was to examine the PCE in the context of the total treatment effect using the RCT data in fibromyalgia.
Methods
A systematic review and meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9].
Data sources and searches
Electronic databases were used for the literature search, specifically Medline (1950–), Web of Science (1960–), EMBASE (1980–), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982–), and Allied and Complementary Medicine (1985–). For Medline, both PubMed and OVID databases were searched. The search was undertaken initially in December 2014 and updated in September 2015 to identify new studies. There were no language restrictions. All results from the database search were exported into EndNote and duplicates were removed. Initially, those with clearly irrelevant titles or abstracts were excluded. If papers could not be excluded via their abstract, full-text copies were obtained and evaluated.
Study selection
There were no age, gender, or ethnicity restrictions. There were also no restrictions on dosage, frequency of delivery, duration of delivery, mode of delivery, or timing of delivery. Studies with relevant clinical outcomes were included, specifically those reporting the Beck Depression Inventory, fatigue score, Fibromyalgia Impact Questionnaire (FIQ) total, visual analogue scale (VAS) pain scale, number of tender points, physical function, and sleep quality. Studies with non-clinical outcomes were not included (Fig. 1).
Only placebo-controlled trials were included to calculate PCE in our main analysis. Any trial in which the treatment or placebo group outcomes worsened or did not change were discarded when calculating the PCE as the negative or zero PCE would not permit log transformation for the meta-analysis [10].
Treatment categories
All medications were categorised according to the British National Formulary (https://www.bnf.org/). These included antidepressants (e.g. duloxetine, amitriptyline, fluoxetine, esreboxetine, citalopram), fibromyalgia agents (e.g. milnacipran), anticonvulsants (e.g. gabapentin and pregabalin) and analgesics (e.g. tramadol, acetaminophen/paracetamol, carisoprodol, and caffeine). All remaining medications were merged into one category as ‘others’ (dolasetron, tropisetron, Nutropin, dehydroepiandrosterone, terguride, pyridostigmine, cyclobenzaprine, interferon alpha). Non-pharmacological treatments included acupuncture, electromagnetic therapy, and other treatment modalities.
Data extraction
Data were extracted by the first reviewer (NW) and validated independently by a second reviewer (AS). Discrepancies were discussed between the two reviewers and any disagreement was discussed with a third reviewer (WZ). The following data were extracted: country in which trial was performed, setting (e.g. community or hospital), number of arms, trial design, presence of industry funding, treatment, type of blinding, presence of allocation concealment, use of intention to treat analysis, duration of treatment, frequency/dose of treatment, diagnosis method, continuation of existing therapy, age, proportion of female participants, duration of fibromyalgia, number of participants, dropout rate, outcome measure description, and results with their standard deviations.
Quality assessment
The RCTs were assessed using the adapted Jadad scale [11]. Allocation concealment (yes/no/unknown) and blinding to patient, physician, and assessor (yes/no) were added in the assessment form to counter the caveats of the Jadad scale.
Data synthesis and analysis
Baseline score, post-intervention score, and change from baseline were entered into the database with their standard deviations. Calculations were undertaken if needed to obtain all these values based on the information provided.
PCE was calculated based on the following formula:
where the numerator presents the contextual effect and the denominator presents the contextual plus specific effects, i.e. total treatment effect. PCE was log-transformed to normalise the distribution. Standard error (SE) of the log(PCE) was calculated using the Hedges method [12]. The standardised mean change from baseline for the treatment group was calculated to present the total treatment effect. In order to calculate the PCE, only improvement of the outcome (e.g. pain relief) was considered. No improvement from baseline (change score = 0) or worsening (negative score) from baseline will be excluded from this calculation. This is because the zero change score or negative score does not permit log transformation of the PCE and it is not a beneficial outcome relevant to the aims of calculating the PCE. It also cannot explain why a treatment worsens the targeted beneficial outcomes unless it is by chance or an error. Therefore, we excluded them from the PCE calculation. A random effects model was used to pool the results with STATA, and the outcomes were stratified according to treatment and other subgroup indicators. I2 index was used to assess heterogeneity [13]. Publication bias was examined using the funnel plot and Egger’s test [14]. Meta-regression analysis (random effects regression) was undertaken if any subgroups showed substantial heterogeneity. The PCE was presented together with the total treatment effect in proportions to demonstrate both contributions from the specific treatment and non-specific contextual effects.
Data availability statement
The dataset(s) supporting the conclusions of this article are available on request from the corresponding author.
Results
Selection of studies
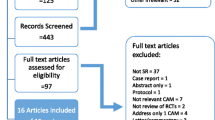
In total, 3912 citations were retrieved from the systematic literature search. Two hundred six duplicates were removed. Two hundred eighty-six potentially eligible studies were identified from reading the abstracts. Full texts were obtained for all 286 papers and 51 gave data for the PCE. A full list of the included studies can be found in Appendix 1.
Characteristics of included studies
Of the 51 studies included in the primary analysis, 46 investigated pain, 30 investigated FIQ total, and 27 investigated fatigue (Table 1).
Proportion of contextual effect
The PCE for all treatments of fibromyalgia was 0.60 (95% CI 0·56 to 0·64) for pain, 0·57 (95% CI 0·53 to 0·61) for FIQ total, and 0·63 (95% CI 0·59 to 0·68) for fatigue score (Table 2). The heterogeneity between treatments varied according to the outcome measured: I2=99.4% for pain, I2=99.2% for FIQ total and I2=97.6% for fatigue. Publication bias was detected in studies for pain and FIQ total (p = 0.014 and p = 0.017, respectively). Figure 2 shows a forest plot for pain outcome.
Figure 3 presents the total treatment effect for pain outcome and the proportion that is attributable to the contextual effect for all treatment modalities. The length of the bar represents the standardised total treatment effect for each treatment category. The percentage is the proportion of contextual effect (blue). Therefore, the remaining part of the bar is the specific treatment effect (red).
Subgroup and meta-regression analyses
Subgroup analyses were carried out in order to examine the predictors of PCE and to explore reasons for heterogeneity. Some variations were observed for pain outcome, e.g. the PCE increased with longer duration of treatment, higher proportion of women, and greater number of participants (Supplementary file 2). However, meta-regression analysis confirmed that none of these factors was a predictor for pain and only duration of treatment > 4 weeks was a predictor for FIQ total (Table 3, Supplementary file 3).
Additional subgroup analysis was undertaken for FDA-approved treatments for fibromyalgia, specifically duloxetine, pregabalin, and milnacipran (www.fda.gov). For pain outcomes, the PCE was 0.66 (95% CI 0.60 and 0.73) for duloxetine, 0.58 (95% CI 0.56 to 0.60) for pregabalin, and 0.81 (95% CI 0.70 to 0.94) for milnacipran. For FIQ score, the PCE was 0.66 (95% CI 0.59 to 0.74) for duloxetine, 0.70 (95% CI 0.68 to 0.73) for pregabalin, and 0.65 (95% CI 0.58 to 0.72) for milnacipran. Forest plots are presented in Supplementary file 4.
Discussion
This is the first study to emphasise the total treatment effect and the proportion of this effect that may be attributable to placebo/contextual effects using the RCT data in fibromyalgia. The study found that the majority of the total treatment effect was contextual (60% for pain, 57% for FIQ, and 63% for fatigue, respectively). This suggests that people with fibromyalgia benefit more from the contextual effects of a treatment than from the specific treatment effect. More interestingly, the proportion varied considerably between treatments, ranging from 44% for electromagnetic therapy and analgesics to 81% for milnacipran (Fig. 3). It is important to recognise that many factors in routine clinical practice influence the magnitude of placebo/contextual response such as patient’s expectations and knowledge of being treated (‘placebo analgesia’ [15]), patient education and patient-doctor interactions (attentive and optimistic ‘positive consultation’, holistic assessment, and desire to follow-up) [16].
This study presents a novel efficacy hierarchy for different treatments based on the total treatment effect and presents the proportion of the total treatment that is attributable to the specific and contextual effects (Fig. 3). This hierarchy has several clinical implications: [1] it informs practitioners about the best and the least effective treatments in fibromyalgia according to overall treatment benefits, which is more likely to accord with their experience in clinical practice than a hierarchy based on specific treatment effects [2]; it helps practitioners to appreciate how important contextual effects are in clinical care for people with fibromyalgia and suggests a way to improve current healthcare delivery through optimisation of modifiable contextual factors while waiting for new ‘stronger’ treatments with high specific effect to be developed [3]; the hierarchy highlights the areas that need further research, such as exercise and other physical treatments, where contextual effect cannot be calculated from RCTs because of the lack of a placebo group due to difficulties in blinding. In addition, this study has found that the PCE increases with the duration of treatment (for pain) and sample size (for FIQ). These data may prove useful firstly for trialists with respect to future design of trials in fibromyalgia, and secondly for clinicians when they manage the disease in clinical practice.
Comparison with other studies
The findings are similar to a meta-analysis of RCTs for osteoarthritis where 75% of treatment effects are explained by contextual effects [6]. The current meta-analysis concurs that the majority of benefits observed in patients are attributed to contextual effects rather than the specific treatment effects. This is not unexpected as both fibromyalgia and osteoarthritis are characterised by chronic pain, restricted function and fatigue, and often show some commonalities in terms of central pain sensitisation [17]. The overall difference in PCE for pain outcomes (60 vs. 75%) between these two conditions may reflect the nature of the disease in that fibromyalgia is often more challenging and difficult to treat than osteoarthritis.
Limitations of study
There are several caveats to this study. Firstly, like other meta-analyses, heterogeneity may affect the outcomes. This is especially true for the primary outcome of pain where the studies collected were highly heterogeneous (I 2 = 99%). Although subgroup analysis was carried out by treatment, high heterogeneity still existed in some treatments such as electromagnetic therapy (Fig. 2). This may be due to the heterogeneity of patients involved in the trials, although the majority of trials in this meta-analysis used the ACR criteria for diagnosis. We were unable to undertake a subgroup analysis based on different diagnostic criteria or subsets so whether PCE varies according to different diagnoses or subsets of fibromyalgia remains to be investigated. Secondly, the number of trials available in each treatment is different and ranges from 2 to 16; hence, the total number of patients involved in each category varied considerably (Fig. 2). Moreover, all studies for electromagnetic therapy or acupuncture were less than 100 participants in size. For small studies, it is not possible to get reasonable estimates of the treatment effect and the proportion that may be attributable to the context. Further, larger trials are needed to stabilise the estimates. Thirdly, apart from duration of treatment, we did not find other predictors of PCE. This is not surprising as we did not have individual patient data to explore the differences in outcome between individual participants, only the differences between trials. Also, we have no information on factors that influence expectancy, which has an important impact on the magnitude of placebo and contextual response. For example, we do not know the extent to which the treatment was explained to participants and whether the information was given in a positive way. Furthermore, we have no information on the illness perceptions and anxiety levels of each participant, whether they have a catastrophizing or positive attitude, or what expectations of treatment benefit they had. It can be assumed that such factors will contribute to participant expectancy and placebo/contextual response and that the overall experience and expectancy will differ between participants receiving the same treatment, even within the same trial. Fourthly, only positive placebo effects were counted in PCE. Negative placebo effects, e.g. nocebo effects, were excluded from the analysis because of inability to log-transform negative results. This may overestimate the contextual effect, but not PCE as the latter is a ratio between placebo and treatment groups where nocebo effects, if there are any, were excluded from both groups under the assumption that treatment consists of active ingredient and placebo. Furthermore, the results have not been compared to studies with a no-treatment group. Therefore, it has been assumed that all the improvements in the placebo group are contextual. Some spontaneous improvement may be due to regression to mean, natural disease fluctuation, or artefact. However, because a proportion was calculated, these spontaneous, non-treatment-related effects should be experienced equally in the active and placebo arms. Under the assumption of the multiplicative model, these spontaneous effects will be cancelled [10].
Conclusion and future research
This study found that a large amount of the treatment effect in fibromyalgia RCTs is attributable to contextual response. Further research needs to be done into what influences the magnitude of this response. With this knowledge, physicians could optimise the context in which they deliver treatment in everyday practice and this would translate into the best possible outcomes for the patient. We suggest that a new hierarchy according to the total treatment effect and the proportion attributable to contextual effects better presents the strength of treatment and will help dispel the efficacy paradox. However, larger, higher quality trials are needed to establish this hierarchy with confidence.
References
Queiroz LP (2013) Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep 17(8):356. https://doi.org/10.1007/s11916-013-0356-5
Rahman A, Underwood M, Carnes D (2014) Fibromyalgia. BMJ: Br Med J 348:g1224. https://doi.org/10.1136/bmj.g1224
Hauser W, Bernardy K, Arnold B, Offenbacher M, Schiltenwolf M (2009) Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum 61(2):216–224. https://doi.org/10.1002/art.24276
Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, Choy E, Kosek E, Amris K, Branco J, Dincer F, Leino-Arjas P, Longley K, McCarthy GM, Makri S, Perrot S, Sarzi-Puttini P, Taylor A, Jones GT (2017) EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 76(2):318–328. https://doi.org/10.1136/annrheumdis-2016-209724
Nuesch E, Hauser W, Bernardy K, Barth J, Juni P (2013) Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: network meta-analysis. Ann Rheum Dis 72(6):955–962. https://doi.org/10.1136/annrheumdis-2011-201249
Zhang W, Zou K, Doherty M (2015) Placebos for knee osteoarthritis: reaffirmation of “Needle Is Better Than Pill”. Ann Intern Med 163(5):392–393. https://doi.org/10.7326/M15-1580
Chen X, Doherty M, Zhang W (2014) THU0329 Placebo effect in fibromyalgia—a systematic review of randomised controlled trials. Ann Rheum Dis 73(Suppl 2):296
Kirsch I, Sapirstein G (1998) Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prev Treat 1(2):2a
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Zou K, Wong J, Abdullah N, Chen X, Smith T, Doherty M et al (2016) Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2015-208387
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12. https://doi.org/10.1016/0197-2456(95)00134-4
Hedges LV, Olkin I. Statistical methods for meta-analysis: Academic press;2014
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ Clin Res Ed 327(7414):557–560
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ Clin Res Ed 315(7109):629–634
Vase L, Riley JL 3rd, Price DDA (2002) Comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain 99(3):443–452. https://doi.org/10.1016/S0304-3959(02)00205-1
Abhishek A, Doherty M (2013) Mechanisms of the placebo response in pain in osteoarthritis. Osteoarthr Cartil 21(9):1229–1235. https://doi.org/10.1016/j.joca.2013.04.018
Haliloglu S, Carlioglu A, Akdeniz D, Karaaslan Y, Kosar A (2014) Fibromyalgia in patients with other rheumatic diseases: prevalence and relationship with disease activity. Rheumatol Int 34(9):1275–1280. https://doi.org/10.1007/s00296-014-2972-8
Acknowledgements
The authors would like to thank the University of Nottingham as a sponsor and host institution of this project.
Contributions
Study concept and design: KZ, MD, and WZ. Data collection and validation: XC and NW ran the literature search. Both reviewers screened relevant full-text articles for inclusion criteria, extracted data for all studies included in the review, and assessed the study quality. KZ and NA (for the first search) and AS (for the second search) reviewed included articles, extracted data and performed the quality assessment independently from the first reviewers (NW, XC). Any inconsistencies were solved by consensus with involvement of a further experienced reviewer (WZ). Data analysis: AS, NW, and WZ. Writing up: NW and AS drafted the paper. All authors contributed to data interpretation and editing of the final paper. All authors approved the final version for publication. WZ is the guarantor of the study.
Funding
This study was supported financially by the Arthritis Research UK Pain Centre (Centre Initiative grant number: 20777). The funding sources had no role in conducting, analysing, or interpreting study results or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Nicola Whiteside and Aliya Sarmanova are joint lead authors
Electronic supplementary material
Supplementary File 1
(DOCX 25 kb)
Supplementary File 2
(DOCX 33 kb)
Supplementary File 3
(DOCX 26 kb)
Supplementary File 4
(DOCX 489 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Whiteside, N., Sarmanova, A., Chen, X. et al. Proportion of contextual effects in the treatment of fibromyalgia—a meta-analysis of randomised controlled trials. Clin Rheumatol 37, 1375–1382 (2018). https://doi.org/10.1007/s10067-017-3948-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3948-3