Abstract
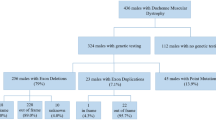
Dysferlinopathies are a group of limb-girdle muscular dystrophies causing significant disability in the young population. There is a need for studies on large cohorts to describe the clinical, genotypic and natural history in our subcontinent. To describe and correlate the clinical, genetic profile and natural history of genetically confirmed dysferlinopathies. We analysed a retrospective cohort of patients with dysferlinopathy from a single quaternary care centre in India. A total of 124 patients with dysferlinopathy were included (40 females). Median age at onset and duration of illness were 21 years (range, 13–50) and 48 months (range, 8–288), respectively. The average follow-up period was 60 months (range, 12–288). Fifty-one percent had LGMD pattern of weakness at onset; 23.4% each had Miyoshi and proximo-distal type while isolated hyperCKemia was noted in 1.6%. About 60% were born to consanguineous parents and 26.6% had family history of similar illness. Twenty-three patients (18.6%) lost ambulation at follow-up; the median time to loss of independent ambulation was 120 months (range, 72–264). Single-nucleotide variants (SNVs) constituted 78.2% of patients; INDELs 14.5% and 7.3% had both SNVs and INDELs. Earlier age at onset was noted with SNVs. There was no correlation between the other clinical parameters and ambulatory status with the genotype. Thirty-seven (45.7%) novel pathogenic/likely pathogenic (P/LP) variants were identified out of a total of 81 variations. The c.3191G > A variant was the most recurrent mutation. Our cohort constitutes a clinically and genetically heterogeneous group of dysferlinopathies. There is no significant correlation between the clinico-genetic profile and the ambulatory status.

Similar content being viewed by others
Data availability
The data that supports the findings of this study are available from the corresponding author on reasonable request.
References
Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet [Internet]. 1998 Sep [cited 2022 Jun 7];20(1):37–42. Available from: https://www.nature.com/articles/ng0998_37
Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet [Internet]. 1998 Sep [cited 2022 Jun 7];20(1):31–6. Available from: https://www.nature.com/articles/ng0998_31
Klinge L, Aboumousa A, Eagle M, Hudson J, Sarkozy A, Vita G, et al. New aspects on patients affected by dysferlin deficient muscular dystrophy. J Neurol Neurosurg Psychiatry [Internet]. 2010 Sep [cited 2022 Jun 9];81(9):946–53. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2975994/
Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R et al (2003) Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423(6936):168–172
Jin SQ, Yu M, Zhang W, Lyu H, Yuan Y, Wang ZX. Dysferlin gene mutation spectrum in a large cohort of Chinese patients with dysferlinopathy. Chin Med J (Engl) [Internet]. 2016 Oct 5 [cited 2022 Jun 7];129(19):2287–93. Available from: https://journals.lww.com/00029330-201610050-00004
Cagliani R, Magri F, Toscano A, Merlini L, Fortunato F, Lamperti C, et al. Mutation finding in patients with dysferlin deficiency and role of the dysferlin interacting proteins annexin A1 and A2 in muscular dystrophies. Hum Mutat [Internet]. 2005 Sep [cited 2022 Jun 27];26(3):283–283. Available from: https://onlinelibrary.wiley.com/doi/10.1002/humu.9364
Polavarapu K, Mathur A, Joshi A, Nashi S, Preethish-Kumar V, Bardhan M et al (2021) A founder mutation in the GMPPB gene [c. 1000G> A (p. Asp334Asn)] causes a mild form of limb-girdle muscular dystrophy/congenital myasthenic syndrome (LGMD/CMS) in South Indian patients. Neurogenetics 22(4):271–85
Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, et al. VarSome: the human genomic variant search engine. Wren J, editor. Bioinformatics [Internet]. 2019 Jun 1 [cited 2022 Jun 29];35(11):1978–80. Available from: https://academic.oup.com/bioinformatics/article/35/11/1978/5146783
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–423
Chakravorty S, Nallamilli BRR, Khadilkar SV, Singla MB, Bhutada A, Dastur R, et al. Clinical and genomic evaluation of 207 genetic myopathies in the Indian subcontinent. Front Neurol [Internet]. 2020 [cited 2022 Jun 1];11. Available from: https://www.frontiersin.org/article/10.3389/fneur.2020.559327
Jacobs MB, James MK, Lowes LP, Alfano LN, Eagle M, Muni Lofra R, et al. Assessing dysferlinopathy patients over three years with a new motor scale. Ann Neurol [Internet]. 2021 May [cited 2022 Jun 1];89(5):967–78. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ana.26044
Krahn M, Béroud C, Labelle V, Nguyen K, Bernard R, Bassez G, et al. Analysis of the DYSF mutational spectrum in a large cohort of patients: DYSF mutational spectrum in a large cohort. Hum Mutat [Internet]. 2009 Feb [cited 2022 Jun 3];30(2):E345–75. Available from: https://onlinelibrary.wiley.com/doi/10.1002/humu.20910
Nalini A, Gayathri N. Dysferlinopathy: a clinical and histopathological study of 28 patients from India. Neurol India [Internet]. 2008 [cited 2022 Jun 16];56(3):379. Available from: http://www.neurologyindia.com/text.asp?2008/56/3/379/40964
Rosales XQ, Gastier-Foster JM, Lewis S, Vinod M, Thrush DL, Astbury C, et al. Novel diagnostic features of dysferlinopathies. Muscle Nerve [Internet]. 2010 [cited 2022 Jun 16];42(1):14–21. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/mus.21650
Dastur RS, Gaitonde PS, Kachwala M, Nallamilli BRR, Ankala A, Khadilkar SV, et al. Detection of dysferlin gene pathogenic variants in the Indian population in patients predicted to have a dysferlinopathy using a blood-based monocyte assay and clinical algorithm: a model for accurate and cost-effective diagnosis. Ann Indian Acad Neurol [Internet]. 2017 Jul 1 [cited 2022 Jun 28];20(3):302. Available from: https://www.annalsofian.org/article.asp?issn=0972-2327;year=2017;volume=20;issue=3;spage=302;epage=308;aulast=Dastur;type=0
Nallamilli BRR, Chakravorty S, Kesari A, Tanner A, Ankala A, Schneider T, et al. Genetic landscape and novel disease mechanisms from a large LGMD cohort of 4656 patients. Ann Clin Transl Neurol [Internet]. 2018 Dec [cited 2022 Jun 1];5(12):1574–87. Available from: https://onlinelibrary.wiley.com/doi/https://doi.org/10.1002/acn3.649
Xi J, Blandin G, Lu J, Luo S, Zhu W, Beroud C, et al. Clinical heterogeneity and a high proportion of novel mutations in a Chinese cohort of patients with dysferlinopathy. Neurol India [Internet]. 2014 Nov 1 [cited 2022 Jul 12];62(6):635. Available from: https://www.neurologyindia.com/article.asp?issn=0028-3886;year=2014;volume=62;issue=6;spage=635;epage=639;aulast=Xi;type=0
Guo QF, Ye ZX, Qiu LL, Lin X, Lai JH, Lin MT, et al. Dysferlinopathy in a cohort of Chinese patients: clinical features, mutation spectrum, and imaging findings. Chin Med J (Engl) [Internet]. 2021 Mar 5 [cited 2022 Jun 3];134(5):622–4. Available from: https://journals.lww.com/10.1097/CM9.0000000000001343
Izumi R, Takahashi T, Suzuki N, Niihori T, Ono H, Nakamura N, et al. The genetic profile of dysferlinopathy in a cohort of 209 cases: genotype–phenotype relationship and a hotspot on the inner DysF domain. Hum Mutat [Internet]. 2020 Sep [cited 2022 Jun 16];41(9):1540–54. Available from: https://onlinelibrary.wiley.com/doi/10.1002/humu.24036
Fernández‐Eulate G, Querin G, Moore U, Behin A, Masingue M, Bassez G, et al. Deep phenotyping of an international series of patients with late‐onset dysferlinopathy. Eur J Neurol [Internet]. 2021 Jun [cited 2022 Jun 1];28(6):2092–102. Available from: https://onlinelibrary.wiley.com/doi/10.1111/ene.14821
Park YE, Kim HS, Lee CH, Nam TS, Choi YC, Kim DS. Two common mutations (p.Gln832X and c.663+1G>C) account for about a third of the DYSF mutations in Korean patients with dysferlinopathy. Neuromuscul Disord [Internet]. 2012 Jun [cited 2022 Jun 16];22(6):505–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0960896612000028
Takahashi T, Aoki M, Tateyama M, Kondo E, Mizuno T, Onodera Y, et al. Dysferlin mutations in Japanese Miyoshi myopathy: relationship to phenotype. Neurology [Internet]. 2003 Jun 10 [cited 2022 Jun 16];60(11):1799–804. Available from: https://www.neurology.org/lookup/doi/10.1212/01.WNL.0000068333.43005.12
Petersen JA, Kuntzer T, Fischer D, von der Hagen M, Huebner A, Kana V, et al. Dysferlinopathy in Switzerland: clinical phenotypes and potential founder effects. BMC Neurol [Internet]. 2015 Dec [cited 2022 Jun 3];15(1):182. Available from: http://bmcneurol.biomedcentral.com/articles/10.1186/s12883-015-0449-3
Acknowledgements
The authors thank the patients and their families who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nashi, ., Polavarapu, K., Bardhan, M. et al. Genotype–phenotype correlation and natural history study of dysferlinopathy: a single-centre experience from India. Neurogenetics 24, 43–53 (2023). https://doi.org/10.1007/s10048-022-00707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-022-00707-3