Abstract
Due to the rapidly increasing price of tungsten carbide and the significant health risks associated with the wear products of WC-Co (Co3O4 and Wo3), an alternative is required. Niobium carbide (NbC) is well suited as a cutting tool due to its high melting point and low solubility in iron. Compared to pure NbC, a complete substitution of WC to NbC-Co resulted in an increased toughness and strength. As alternative binders, nickel and iron-based binders were subsequently investigated. Although iron-based cermets would be an economical, low-cost alternative to NbC-Ni cermets, they showed a higher coefficient of friction and wear rate. So far, NbC-Ni cermets best met the requirements of high hardness and toughness. Various secondary carbides such as VC, Mo2C, TiC, but also WC were added to further improve the hardness. Elemental analyses of NbC-Ni-MeC cermets (Me = metal) showed that the binder is a face-centered cubic solid solution, while the NbC phase is a solid solution of the type (Nb, Me)C.
Zusammenfassung
Aufgrund des rasanten Preisanstiegs von Wolframcarbid und signifikanter Gesundheitsrisiken, die mit den Verschleißprodukten von WC-Co-Hartmetallen (Co3O4 and Wo3) einhergehen, wird eine Alternative benötigt. Niobcarbid (NbC) ist als Zerspanwerkzeug aufgrund seiner hohen Schmelztemperatur und seiner geringen Löslichkeit in Eisen gut geeignet. Im Vergleich zu reinem NbC, führte eine vollständige Substitution von WC zu NbC-Co zu einem Anstieg der Zähigkeit und Festigkeit. Als alternative Binder wurden anschließend Nickel- und Eisengebundene Binder untersucht. Obwohl eisengebundene Hartmetalle im Vergleich zu NbC-Ni Hartmetallen eine ökonomische und kostengünstigere Alternative darstellen würden, zeigten sie einen höheren Reibkoeffizienten und eine höhere Verschleißrate. Bisher erfüllen die NbC-Ni-Hartmetalle die Anforderungen hinsichtlich einer hohen Härte und Zähigkeit am besten. Verschiedene Sekundärkarbide, wie VC, Mo2C, TiC, aber auch WC wurden zusätzlich hinzugefügt, um die Härte weiter zu steigern. Elementanalysen der NbC-Ni-MeC Hartmetalle (Me = Metall) zeigten, dass der Binder eine kubisch-flächenzentrierter Mischkristall ist, während die NbC Phase ein Mischkristall des Typs (Nb, Me)C ist.
Similar content being viewed by others
1 Introduction
Cemented carbides are metal matrix composites (MMCs) consisting of a hard carbide phase, usually tungsten monocarbide (WC), which is embedded in a ductile metallic binder. In cemented carbides and cermets the hard carbide phase is dominant, whereas in MMCs the binder (or the matrix) is the dominating phase. The term “cermet” is composed of the terms “ceramic” and “metal” and describes a subgroup of cemented carbides, where the dominating hard carbide phase is not WC, but TiC or NbC embedded in a Co, Ni or Fe binder matrix. So far, tungsten carbide (WC) has been the first choice as cemented carbide, as it accounts for 70% of the cutting tools produced [1]. However, the wear products of WC-Co, namely Co3O4 and WO3 [2,3,4], are filed in the framework of the European “Registration, Evaluation, Authorization and Restriction of Chemicals” (REACH) [5] and pose health risks.
Niobium carbide (NbC) is biocompatible and has a low solubility in iron, cobalt, and nickel, which is beneficial in hot tribological contact. Due to its high melting temperature, NbC has a longer service life at high temperatures, which is accompanied by a reduction of adhesive wear compared to WC [4]. In addition, no hazards, or critical notifications for Nb2O5 and NbC are filed in the REACH and the global reserves of niobium exceed those of tungsten by at least one order of magnitude. Table 1 summarizes the most important properties of WC and NbC for comparison. It should be kept in mind that the properties of NbC1−x vary with the homogeneity range [6].
In 1968, Warren published the first study about the microstructural development of NbC-Co during liquid phase sintering (LPS) [11]. In the following year, Warren examined the carbide grain growth during LPS in NbC cemented carbides with Fe, Co, or Ni binder [12]. Since melting processes face problems of sedimentation or floating of the hard phase particles in the molten binder matrix [13] and the dissolution of the particles in the melt [14], cemented carbides are commonly processed by powder metallurgy techniques including different sintering techniques.
NbC cermets as cutting tools are a relatively new research field. In 2001, Moon et al. [15] investigated the grain growth of NbC-Co cermets during liquid phase sintering (LPS). In 2007, Huang et al. published two studies on the mechanical properties of NbC-Co cermets concerning the influence of WC addition [16] and the influence of spark plasma sintering (SPS) [17]. With a density of 7.82 g/cm3, NbC is more cost effective than WC, since only ca. half the mass of NbC is needed to produce NbC cermets compared to WC cemented carbides. In addition, thermal stresses induced between NbC and metallic binder are lower than for WC, due to a more similar coefficient of thermal expansion and lower Young’s modulus of NbC. Several different approaches to make NbC cermets compatible to WC cemented carbides led to the investigation of different manufacturing processes and the use of binders and binder additives, namely secondary carbide phases. Investigations were carried out with Co, Ni, and Fe3Al as binders and VC, Mo2C, WC, TiC, and Cr3C2 as secondary carbides.
The aim of this review is to provide an overview of the previous studies on NbC cermets, focusing on different binder and the addition of secondary carbides. Besides outlining the current state of research in this field, new research approaches are suggested. The review highlights the advantages of NbC and its cermets and shows that, despite the short development time, the performance of NbC cermets is in certain applications already better than that of WC cemented carbides. The effect of different binders and of different secondary carbides on microstructure and mechanical properties is discussed.
2 Niobium carbide
Niobium carbide (NbC) is a group 5 transition metal carbide and exhibits several short and long range ordered phases, which occur depending on the C content. The reader is referred to Cuppari and Santos [18], who give an overview of the structure and properties of NbC in their review. The combination of different types of chemical bonds including covalent, metallic and ionic bonds makes it suitable for various applications, i.e. as cutting and grinding tool [19] but also in the electronics industry as electrical contact coatings [20], for diffusion-resistant thin-film coatings of microcircuit devices [21], and for nanocomposite films (nanocrystalline NbCx/amorphous carbon) [20]. Further applications of NbC as inexpensive electrode material in Li-ion and Na-ion rechargeable batteries are fuel cells and electrolyzers. In addition, NbC can also be employed for electrochemical supercapacitors and for electrochemical reactions such as hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), and oxygen evolution reaction (OER) [22]. For all these applications, it is important to ensure high performance and long component life through optimization of several properties such as hardness, heat stability, fracture toughness, strength, and wear resistance [23].
3 NbC cermets
Since binderless NbC is brittle, cermets and metal matrix composites combine advantages for example of the hardness of ceramics and the fracture toughness of the binder. Extensive parameter studies were performed to investigate different metal bonded NbC cermets including cobalt-, nickel-, and iron-based binder. The binder content is either given by weight percentage (wt%) or volume percentage (vol%).
3.1 Powder metallurgical processing
Different powder processing routes were used to produce NbC cermets. NbC shows a poor sinterability [18], therefore sintering temperatures between 2000 °C and 3000 °C are necessary. By applying pressure, the sintering temperature can be reduced below 1500 °C while overcoming the poor sinterability of NbC. This is usually done through hot pressing (HP) and spark plasma sintering (SPS) [17, 19]. Furthermore, the densification is influenced by the diffusion rate and wetting behavior of the binder, its proportion to the hard carbide phase, and the solubility of the carbide phase as well as of additional carbide particles in the binder [23, 24]. Therefore, each processing route has its own advantages depending on the amount of NbC and the binder phase, affecting the microstructure and thus the mechanical properties [23, 25]. Different powder metallurgical routes are shown in Fig. 1 and some are explained in more detail below.
In every case, the NbC hard phase and the binder powder as well as additional ceramic particles must be pre-mixed to achieve an almost uniform distribution of all powders. One method to produce dense cermets is colloidal processing as reported by Wäsche et al. [26]. An aqueous suspension of the powder is produced through non-magnetic agitating. The suspension is further homogenized through pulsed ultrasound at room temperature and frozen down to −30 °C in a freeze dryer. The powder mixture is dried and subsequently milled to break up agglomerates. After milling, the powder is uniaxial pressed to green densities of 55 to 60%. The so-called green bodies are finally sintered through gas pressure sintering (GPS). The metal powder is thereby in the liquid state, which is called liquid phase sintering (LPS). Additionally, during GPS, the pressure is increased at the end of the holding time, leading to densities above 99.5%.
An established processing route to produce dense materials is mechanical milling followed by pressing, LPS and hot isostatic pressing (HIP). Mechanical milling is thereby employed to distribute the metal powder particles uniformly around the carbide particles. As with GPS, the pressure is increased to eliminate surface porosity [23]. The difference between GPS and LPS + HIP is the pressure applied, which can be increased up to 2000 bar with HIP, while it is significantly lower with GPS. If only LPS is employed, a remarkable grain growth is observed. In most studies concerning NbC cermets, LPS is compared to either hot pressing (HP) or spark plasma sintering (SPS) [16, 23, 27], because both HP and SPS can significantly suppress grain growth [17].
Hot pressing (HP) of NbC cermets is often carried out under vacuum and pressures between 45 and 50 MPa [16, 28] at temperatures up to 1300 °C for 10 min. However, to consolidate binderless NbC, the temperature must be increased to 2150 °C [28]. Using SPS, advantages of HP are combined with significantly faster heating and cooling rates, namely 100 K/min to 200 K/min compared to 20 K/min to 40 K/min. This is the reason why SPS is the fastest way of sintering. SPS is also known as field assisted sintering technique (FAST) or pulsed electric current sintering (PECS).
Fig. 2 shows exemplarily a SPS cycle of a NbC-Co cermet. In contrast to HP, heat generation is internal, which is the reason why the sintering temperature can be significantly reduced [29]. However, higher internal stresses are likely to be retained during SPS due to the high cooling rate. Through SPS, higher hardness and lower fracture toughness KIc can be achieved compared to LPS [30], which makes the hardness of spark plasma sintered NbC cermets comparable to cemented wolfram carbides [4]. Since SPS is not widely employed in the industry yet, GPS and LPS (+HIP) offer the advantage of instantly scaling the processing route.
Temperature and pressure versus time during SPS of NbC-Co cermets for 2 min at 1280 °C under a pressure of 30 or 60 MPa. Reprinted from Materials Letters, 61, S. G. Huang, O. Van der Biest, L. Li, J. Vleugels, Properties of NbC-Co cermets obtained by spark plasma sintering, 574–577, Copyright (2007), with permission from Elsevier [17]
After processing, the electrical conductivity of NbC makes it possible to use electrical discharge machining (EDM) [19] to cut out different sample shapes, as shown in Fig. 3. The obtained samples can be employed for various tests such as 4‑point-bending and elastic modulus tests (top left), machining tests (cutting inserts, top right), and tribological tests, namely continuous (bottom left) and oscillating sliding (bottom right).
Different samples cut out through elecrical discharge machining from disks for: a mechanical testing, and b cutting inserts; c for continuous and d for oscillating sliding: Reprinted with permission from the Metal Powder Industries Federation, 105 College Road East, Princeton, New Jersey, USA [31]
3.2 NbC-Co cermets
First fundamental studies on NbC grain growth in a Co matrix during sintering were conducted in 1968 by Warren [11] and in 2001 and 2004 by Moon et al. [15] and Cho et al. [32], respectively. Since WC cemented carbides are mainly produced and used with Co-binder, namely WC-6Co, Co bonded NbC cermets, containing binder contents of 8 wt% or 12 wt% Co, have been produced [8].
3.2.1 Microstructure
Warren investigated the growth and change in shape of NbC grains during liquid phase sintering (LPS) [11]. He assumes that grain growth occurs by solution and/or precipitation processes, which are controlled by diffusion. The C/Nb ratio, however, has no significant effect on grain growth. Small alloying additions of WC, TiC, and NbB2 inhibit grain growth and/or alters the growth process affecting the grain shape.
Further investigations indicate that TiC changes the solution behavior of NbC in Co binder and affects decisively the interface reaction. Entrapped Co binder in NbC grains indicate grain coalescence in NbC-Co-TiC cermets. While the grain growth mechanisms are not affected by the addition of WC and/or TiC, the addition of NbB2 leads to the formation of a eutectic phase within the binder phase due to the inhibition of NbC solution and the enhancement of interfacial diffusion. Moon et al. [15] studied the growth mechanisms of round edged NbC grains in Co binder and assume that these grains are the equilibrium shape and result from Ostwald ripening. According to Ostwald ripening, large grains have the shape of a cube with slightly rounded edges, while small grains are almost spherical. These findings confirm the assumptions of Warren that grain growth is diffusion controlled. However, differences between spherical and cubic NbC grains occur.
Moon et al. [15] find that the rate of grain growth increases with increasing fraction of rough interface area. This indicates that faceted grain with rounded edges grow diffusion driven and the roughness of the edges controls the overall growth kinetics of the system, leading to a lower growth rate with increasing curvature of the edges (grain growth retarding effect). Cho et al. [32] investigated the influence of temperature on the grain growth and grain shape during LPS and observe a gradual change from abnormal to normal growth as the temperature approaches the surface roughening transition temperature, at which grains are completely spherical. Furthermore, the researchers verify that the surface roughening transition is reversable by an additional heat-treatment, which leads back to the “initial” grain shape. In comparison to binderless hot pressed NbC, the particle size d90 of the spark plasma sintered NbC-Co is reduced from d90 = 18.12 µm to d90 = 3.78 µm [19].
Another behavior is observed with addition of WC as secondary carbide phase. Up to a content of 10 wt% WC, the grain size of NbC slightly increases, while the grain size of NbC decreases with WC contents above 10 wt%. Huang et al. describe their observation by the dissolution of up to 10 wt% WC in both NbC and Co binder, resulting in the formation of core-rim-structures [16]. The researchers find that the W‑enriched rim structure is less pronounced when the cermet is sintered through LPS compared to HP. Additionally, with increasing sintering temperature, the grain morphology change from facetted to spherical, which corresponds to previous observations of Cho et al. [32].
Huang et al. [16] investigated the influence of WC content on the lattice parameter of NbC and the (Nb,W)C phases. With increasing dissolution of WC into the NbC grains, the lattice parameter of the pressureless sintered cermet decreases due to the replacement of larger Nb by smaller W atoms. During hot pressing, the lattice parameter remains constant above a WC content of 10 wt%, which indicates that no further diffusion has taken place and that saturation of WC in NbC has been reached. Due to the limited solubility of WC in NbC, in NbC cermets with higher WC contents, not dissolved WC grains act as diffusion barrier and pin grain boundaries, leading to a limited NbC grain growth.
3.2.2 Properties
Fig. 4a shows the Vickers hardness versus the indentation load of HP-NbC, NbC-8Co and NbC-12Co. The microhardness of NbC-8Co and NbC-12Co significantly decreased compared to hot pressed binderless NbC (HP-NbC) [19]. At low indentation loads (HV 0.2), pure NbC has a significantly higher microhardness than NbC-Co cermets, while above HV2, the microhardness is in the range of NbC-Co cermets. Woydt and Mohrbacher [33] explain this difference in hardness with the higher plastic deformation of NbC-Co cermets at room temperature due to increased dislocation movement under indentation.
Vickers hardness of NbC-Co cermets: a as a function of indentation load, additionally for binderless NbC (adapted from [33]); b versus fracture toughness KIc, with different secondary carbides. The dashed line marks the benchmark of WC, while the solid line marks the benchmark of commercial cermets (adapted from [10, 34])
In a further study, Huang et al. [10] investigate the influence of the sintering process, namely conventional sintering (CS) and spark plasma sintering (SPS), and the addition of secondary carbide phases on NbC-12 wt% Co cermets, which is shown in Fig. 4b. SPS lead to a higher Vickers hardness than conventional sintering, while the fracture toughness tends to decrease, which is attributed to the smaller grain size of the spark plasma sintered cermets.
The addition of secondary carbides, namely VC, Mo2C and WC, significantly influences hardness and fracture toughness. VC and Mo2C increase hardness independent on the sintering process. However, fracture toughness increases through the addition of Mo2C in case of CS, while toughness decreases through SPS. The addition of WC significantly increases the fracture toughness of CS cermets, while it decreases that of SPS cermets without increasing the hardness through both sintering processes. Therefore, WC does not seem to be as applicable as other secondary carbides in NbC-Co cermets to increase both hardness and fracture toughness.
A combination of Ni and Co as binder show a significant increase in fracture toughness through SPS, which is reported by Woydt et al. [34]. In a recent study, Huang et al. [35] investigated the influence of WC and Ti(C0.7N0.3) on NbC-NiCo cermets. However, they did not investigate the effect of NiCo content on hardness and fracture toughness. To the best of our knowledge, no further studies are published investigating the NiCo binder.
Regarding hot hardness, the comparison of WC cemented carbides with NbC-Co cermets shows that the decrease in hot hardness is less pronounced for NbC-Co than for WC-Co, which might be due to the higher melting temperature of NbC leading to a higher thermal stability. Woydt and Mohrbacher [36] report that at 600 °C NbC0.88-12Co has the same hot hardness as WC cemented carbides, while at 700 °C, NbC0.88-12Co shows a higher hot hardness. This is beneficial for applications at high temperature.
Fig. 5 shows Young’s (E) and shear (G) moduli versus temperature of HP-NbC, NbC-8Co, and NbC-12Co. Please note that Woydt et al. [33] report on the “bulk modulus G”. Regarding the properties of NbC in Table 1 however, we assume that the authors meant the shear modulus G, which is the reason why we refer to the shear modulus in the following. HP-NbC shows the highest moduli, followed by NbC-8Co and NbC-12Co.
Influence of temperature on Young’s (E) and shear (G) moduli of hot pressed (HP) NbC and spark-plasma sintered (SPS) NbC-8Co and NbC-12Co. (Adapted from [33])
While no large difference is observed in Young’s modulus, the shear modulus of HP-NbC is significantly larger than that of NbC-8Co and NbC-12Co. With increasing temperature up to 800 °C, Young’s moduli decrease by about 40 GPa to 70 GPa, while shear moduli decrease only 20 GPa to 30 GPa. Therefore, the NbC-Co cermets are more sensitive to shear stress than pure NbC, while the response to linear stress is similar for all samples. The decrease in both E and G with increasing temperature is, however, more pronounced for the Young’s moduli.
3.3 NbC-Ni cermets
Since the use of Co involves health issues and the substitution of WC by NbC lead to a relatively poor wettability of the binder [17, 37], Co is consequently substituted with Ni binder. NbC-Ni cermets show improved toughness but reduced hardness compared to NbC-Co cermets [4]. The addition of carbon, molybdenum or aluminum to the binder and the influence of sintering temperature and of secondary carbides such as VC, Mo2C, TiC, and WC on NbC-Ni cermets were recently investigated. All sintered cermets have in common that the Ni binder phase is composed of a fcc solid solution and the NbC phase is a cubic solid solution of the type (Nb, Me)C regardless of the sintering temperature [38].
3.3.1 Influence of carbon content
The carbon content influences several aspects in a metal bonded cubic carbide cermet system such as hardness, grain size, the morphology of the hard phase, and the composition of the binder phase [39]. Huang et al. investigated the influence of C on NbC-12Ni (vol%) [39]. In NbC-12Ni cermets, rapid grain growth of NbC can occur up to grain sizes of 30 µm [38]. By adding NbH2 or C to the powder mixture during processing, the NbC grain size can be significantly changed [39]. The addition of C increases the C content of the NbC phase but reduces the lattice parameter, whereas the addition of NbH2 has the opposite effect and leads to smaller NbC grain sizes than in the carbon-added cermets. Furthermore, the solubility of Nb in the Ni binder increases with decreasing C/Nb ratio of the NbC phase. With increasing Nb solubility, the lattice parameter increases.
Huang et al. [39] reported a significant decrease in Vickers hardness (HV30) and fracture toughness with increasing carbon content, which might be due to free graphite within the microstructure. An increase in hardness is further attributed to the hardness of the NbC itself and the NbC grain size. Nonetheless, the effect of the addition of NbH2 and C depends on the C content of the NbC starting powder itself.
3.3.2 Influence of aluminum content
The first and only study about the influence of aluminum in Ni binder was published by Huang et al. in 2016 [40]. The researchers show that the addition of 30 mol% Al powder to the Ni binder lead to grain refinement with sizes smaller than 10 µm. The microstructural characterization of the dissolved Al in the Ni binder showed a homogenous distribution of Al, but also Al2O3 grains. Aluminum reduces the driving force for NbC dissolution-reprecipitation, which may be one reason why Al2O3 grains are detected; however, Huang et al. assume that the oxidation of Al metal powder during powder mixture preparation is the main reason. Investigating the fracture toughness, the researchers observe a lower fracture toughness but higher hardness for the Al-doped NbC-Ni cermets, due to the intrinsically low room temperature ductility of the Ni binder.
3.3.3 Influence of molybdenum content
Huang et al. investigated the influence of Mo on C‑rich NbC-12Ni cermets [39]. Based on XRD patterns, the lattice parameter of the (Nb,Mo)C mixed carbide decreases with increasing Mo content, while the lattice parameter of the Ni binder increases in the NbC-Ni cermet. Huang et al. explain this finding with the dissolution of Mo in the Ni binder and in NbC, which is characterized by the replacement of Mo atoms by Nb atoms [39]. By adding up to 15 vol% Mo to the NbC, graphite grains disappear and a fine NbC grain (< 5 µm) microstructure is obtained. Elemental analysis prove that no metallic Mo or Mo2C were detected in these cermets, which is due to a higher solubility of Mo in the Ni binder than in the carbide phase [4]. Solid solution (Nb,Mo)C carbides form with a reduced grain size.
In TiCxNy-based cermets, it is already known that Mo and Mo2C refine the ceramic grains [39]. Due to the reduced grain size of the mixed (Nb,Mo)C carbide, NiMo-bonded NbC cermets compensate for losses in hardness while the toughness remains unchanged [4]. Moreover, while the hardness HV30 increases with increasing Mo content, the toughness decreases up to a Mo content of 10 vol%, which is attributed to the NbC grain refinement, the formation of solid solution (Nb,Mo)C carbides and the decreased C content in the mixed carbide [39].
3.3.4 Influence of secondary carbides
Besides the influence of C/Nb ratio and the addition of Mo or Al to the Ni binder on the properties of NbC-Ni cermets, the addition of secondary carbides can significantly alter the microstructure, and thus the properties [39]. To achieve a good chemical bond between Ni binder and NbC, and thus a good load-bearing capacity, the wetting behavior is important. A good wetting behavior is related to the enthalpy of formation ∆Hf and can be achieved both by increasing the carbon content and by adding secondary carbides such as Mo2C, VC, WC and TiC [38, 39]. Table 2 summarizes the ∆Hf of NbC and the monocarbides. Mo2C has the lowest enthalpy followed by WC, VC and NbC [41]. However, in our opinion, WC should be avoided in NbC cermets due to the known health risks.
Fig. 6 shows the influence of VC and Mo2C secondary carbides on the Vickers hardness and fracture toughness of NbC-Ni cermets. The dashed line marks the benchmark of WC. With increasing binder content from 12 to 20 vol%, the fracture toughness significantly increases, whereas it deceases with excessive addition of VC and Mo2C [42]. In general, the addition of VC and Mo2C as secondary carbides increases the Vickers hardness. In comparison to NbC-Ni-Mo2C, NbC-Ni-VC cermets have a higher fracture toughness, which makes them better than WC cemented carbides considering the ratio of KIC to hardness.
Influence of secondary carbides on Vickers hardness and fracture toughness of NbC-Ni cermets. The dashed line marks the benchmark of WC. (Data from [42])
Further, Woydt et al. [34] recommend substituting stoichiometric NbC1.0 by a sub-stoichiometric NbC0.88. As with the Co-bonded NbC cermets, the hardness of the NbC and thus the hardness of the NbC-Ni cermets increases, which counteracts the loss of hardness due to the Ni binder. However, no further studies have been published on this subject, which may indicate that secondary carbides have proven to be more effective in increasing the hardness than the NbC0.88.
Mo2C content
Huang et al. investigated the influence of the addition of up to 15 vol% of Mo2C on NbC-Ni cermets produced by LPS [42]. Due to the smaller atomic radius of Mo, the lattice parameter of the solid solution (Nb, Mo)C decreases with increasing Mo2C content. The lattice parameter of the Ni binder, however, increases with increasing Mo2C content, which was also reported by Kayser [43], who investigated the influence of Mo in Ni-Mo alloys on the lattice parameter. Kayser observed small volumes of a Ni-Mo β phase in samples above 15 at% Mo, which would explain line broadening observed in the XRD investigations. Neither Kayser nor Huang et al. explicitly explained the increase in lattice parameter. Since line broadening is influenced by lattice defects and can indicate an increase in lattice strain, we assume that the β phase precipitation might be a reason for the increasing lattice parameter of the Ni binder.
The influence of Mo2C addition (3, 6 and 9 vol%) on the sintering behavior and microstructural evolution on NbC-12Ni‑0.5WC cermets was recently investigated by Labonne et al. [44]. In this study LPS under vacuum was deployed. EDS elemental analysis showed Ni segregation, which was estimated to be about one Ni monolayer at the NbC grain boundaries, indicating good wetting behavior of the Ni binder. Furthermore, Mo2C particles delay the shrinkage of the material during the solid-state sintering to higher temperatures, but densification is completed after LPS [44].
The lattice parameter behaves as with pure Mo addition, because Mo2C dissolves in NbC to form (Nb,Mo)C solid solution carbides. This is also the reason why Huang et al. [38] find a decreasing grain size from 2.19 µm to 1.78 µm with addition of Mo2C. Labonne et al. [44] further performed XRD of interrupted sintering tests, which revealed that WC and Mo2C almost completely dissolved between 1080 °C and 1180 °C during the solid-state sintering. While W is mostly detected in the binder above 1180 °C, Mo is dissolved in both NbC and Ni binder. The recent study of Huang et al. [45], who investigated the influence of up to 15 vol% Mo2C on NbC-Ni cermets, confirms this finding. The maximum solubility of Mo2C is 33 wt%, which explains the complete dissolution of Mo2C in NbC cermets, where (Nb,Mo)C solid solution and Ni alloy binder have a cubic structure [45]. Further, an increase in hardness with Mo2C addition is observed, which is mainly attributed to grain refinement, while fracture toughness decreased [44, 45].
VC content
The addition of VC in NbC-Ni cermets was intensively investigated by Huang et al. [38, 39, 42]. VC significantly inhibit NbC grain growth and lead to a homogeneous grain size distribution, as Mo and Mo2C. As with the NiMo-bonded NbC cermets, solid solution carbides (Nb, V)C are formed with grain sizes below 10 µm, which were significantly reduced in size with increasing VC content [42]. Huang et al. also reported that the decrease in grain size is due to the increasing dissolution of VC, which causes a decrease in the lattice parameter both in NbC and Ni binder and affects the surface energy. Additionally, no contrast is observed within the (Nb, V)C grains, indicating a homogeneous distribution of vanadium. In general, the addition of VC is more effective than Mo2C to increase the fracture toughness [42].
The C/Nb ratio influences the Vickers hardness and fracture toughness of NbC-12Ni and NbC-12Ni-5VC cermets. With decreasing ratio, the hardness increases in both cermets. Overall, the NbC-12Ni-5VC cermets show a higher hardness, which is related to the grain refinement and formation of (Nb,V)C solution carbide due to the VC addition. As the hardness, fracture toughness increases with decreasing C/Nb ratio in NbC-12Ni cermet. In case of NbC-12Ni-5VC, a maximum fracture toughness is observed at a ratio of ca. 0.94. Down to a sub-stoichiometry of 0.92, the cermet with VC addition has a higher KIC than the NbC-12Ni cermet, which is the opposite below a C/Nb ratio of 0.9.
Huang et al. additionally studied the effect of the sintering temperature on the microstructure of NbC-12Ni-4Mo-4VC cermets [38]. At temperatures below T ≤ 1340 °C, residual porosity is observed as well as Ni binder between the NbC grains. At temperatures above T > 1380 °C, NbC-Ni cermets are fully densified and have a homogeneous distribution of cubic NbC grains and Ni binder phase. Fig. 8a shows the influence of the sintering temperature on the average grain size. Rapid grain growth occurs at temperatures above 1420 °C, leading to NbC grains up to 30 µm and an average grain size of up to 2.2 µm. However, the average grain size is significantly reduced to 7.7 µm compared to NbC-Ni without additives. Huang et al. explain this effect by the inhibition of grain growth due to the addition of Mo and VC. Fig. 7a shows the influence of the sintering temperature on the lattice parameters of the NbC phase and binder phase, which were determined from XRD patterns using the Rietveld’s structure refinement analysis. At higher temperatures, Mo and VC dissolve, which lead to a decrease in lattice parameter of NbC and Ni binder and to an increase in grain growth. Dissolution of VC and Mo in Ni binder additionally decreases the melting temperature resulting in a significant shrinkage during densification, which is initiated in the solid-state of the binder. Fig. 7b shows the influence of the sintering temperature on the NbC grain size of NbC-12Ni-4Mo-4VC. The correlation of the Ni lattice parameter with the NbC grain size is due to the formation of solid solution of (Nb, Mo, V)C and the dissolution of Mo and V in the Ni binder. Huang et al. additionally attribute residual thermal stresses during sintering and cooling to the change in lattice parameter [38].
Influence of the sintering temperature on: a the lattice parameter of NbC and Ni binder, and b the average grain size of a NbC-12Ni-4Mo-4VC cermet. (Adapted from [38])
Influence of the sintering temperature on the microhardness (HV30) and fracture toughness (KIc) of NbC-12Ni-4Mo-4VC cermets. (Adapted from [38])
Fig. 8 shows the effects of the microstructure on the microhardness and fracture toughness on the NbC-12Ni-4Mo-4VC cermets depending on the sintering temperatures. The increase in hardness relates to a decrease in grain size and vice versa [46]. The hardness reaches its maximum at T = 1380 °C corresponding to the lowest solid solution grain size of 1.59 µm. In contrast, the decrease in hardness with increasing sintering temperature is attributed to grain coarsening, while the increase in fracture toughness shows more complex dependencies, which could be a reason why the structure-property relationships are not further explained by Huang et al. [38]. Huang et al., however, report that an excessive addition of VC (> 5 vol%) and Mo2C (> 10 vol%) results in a decrease in fracture toughness [38]. Moreover, the addition of secondary carbides leads to cracks mainly propagating along the carbide and binder grain boundaries (VC cermets), whereas trans-granular crack propagation is the dominating behavior observed in the pure NbC-Ni cermets [39]. Huang et al. further report trans-granular cracks with twist hackles, which twist and turn to follow preferred cleavage planes within the microcrystals and contribute this finding to the increase in fracture toughness of the NbC-12Ni5VC cermet [42].
TiCx and TiCxNy content
Huang et al. [38] investigated the influence of the addition of TiC particles on the microstructure of NbC-Ni cermets. Elemental mapping revealed core-rim structures of cubic NbC grains embedded in fcc Ni matrix. The researchers assume that undissolved NbC act as nucleation site for the rim structure resulting in a mixed carbide core of (Nb,Ti)C and a rim area. The rim area thereby contains higher Ti concentrations in the inner than in the outer rim.
Huang et al. [35] recently investigated NbC-Ni cermets with WC and TiC0.7N0.3 addition, which were produced by LPS. The researchers find that NbC cermets would always form rimless Ti(C,N) grains and core-rim structured NbC grains due to the solubility of the secondary carbides in the binder and the high difference in lattice parameter of NbC and Ti(C,N). In a further study, Huang et al. add Ti(C0.7N0.3) to an NbC-Ni-VC cermet and observe that Ti(C0.7N0.3) contents below 15 vol% exhibit a fcc solid solution Ni binder and a cubic core-rim solid solution phase, namely (Nb,V,Ti)C [47]. Above a Ti(C0.7N0.3) content of 25 vol%, the researchers observe that 3.8 vol% of the particles remained undissolved, while the other Ti(C0.7N0.3) particles completely dissolved within the NbC and binder phases.
In NbC-NiCo cermets, Huang et al. [48] report a similar finding. A complete dissolution of WC and Ti(C0.5N0.5) secondary carbides is observed as well as the formation of core-rim structures during sintering. With increasing amount of Ti(C0.5N0.5), the thickness of the rim of NbC solid solution grains increases, while the partial substitution of Ni by Co in the binder do not affect the rimless structure of N‑rich Ti(C,N) grains in cermets with contents of Ti(C0.5N0.5) above 22 wt%. These findings are supported by Ettmayer et al. [49], who report a higher solubility of substances with lower surface energy in molten metal. According to the researchers, the solubility of NbC, TiC, VC, WC, and Mo2C in Ni at a temperature of 1400 °C is 7, 11, 14, 27, and 36 wt%, respectively. Thus, TiC has a higher solubility in NbC than VC and Mo2C, which is the reason why it acts less efficiently as barriers against NbC grain growth.
Huang et al. [48] additional suggest that as with Ti(C,N) based cermets, the core-rim structure can be altered through the N content of the starting powder or the use of N2 as sintering atmosphere and a high carbon activity when using Ti(C0.7N0.3). They observe significant lower grain sizes of less than 1 µm compared to the Ti(C0.5N0.5)-free NbC cermets, which is attributed to N as grain growth inhibitor.
As known from the addition of other secondary carbides, the addition of TiC or Ti(C0.7N0.3) enables to control the grain growth in NbC cermets, as shown in Fig. 9a. However, Huang et al. [38] do not explain the increase in grain size in the NbC cermets with addition of 25 vol% Ti(C0.7N0.3). The overall smallest grain size of 0.94 μm in an NbC-Ni cermet with TiC addition is observed for the composition (NbC-10TiC)-7.5 Mo2C‑12 Ni.
Influence of TiC0.7N0.3 content on a the grain size and b hardness and fracture toughness of NbC-10Ni‑7.5VC cermets. (Data from [47])
The researchers report a significant influence of the secondary carbide content on the phase content within the cermet. Up to a WC content of 34 wt%, hardness and toughness changes only gradually. Above this value, the lattice parameter of the binder as well as the hardness decrease dramatically, while the toughness increases [35].
The influence of the TiC0.7N0.3 content on microhardness and fracture toughness of NbC-10Ni‑7.5VC cermets is shown in Fig. 9b. With increasing TiC0.7N0.3 content, both microhardness and fracture toughness increase. Huang et al. [47] attribute the increase to the reduced NbC grain size, the formation of solid solution carbides, and the change in C/Nb ratio in the mixed carbide. With the addition of TiC nanoparticles in NbC-Ni cermets produced by GPS [26], a NbC-TiC solid solution forms as hard phase and Ni-Nb as metallic binder phase with a Nb content of 3 wt%, causing the formation of additional graphite. The hardness shows no significant dependence on the TiC content. However, the hardness decreased using gas pressure sintering compared to vacuum sintering, which is attributed to the graphite formation on NbC-TiC grain boundaries.
WC content
Huang et al. [45] investigated the influence of up to 15 vol% WC on NbC-Ni cermets. As with Mo2C addition, the cermets contains of a (Nb,W)C solid solution and Ni alloy binder as cubic phase and residual WC above 10 vol% WC addition. However, the researchers calculated a maximum solubility of 23 wt% WC. The solid solution phase consists of a Nb-rich core and a WC-richer rim, which is more pronounced with increasing WC content. Microstructural characterization shows black spots, which are pores or oxygen rich NbCxOy. The shape of the (Nb,W)C grains changes from faceted with rounded corners to a rounded with increasing WC content. The Vickers hardness slightly increases but remains unchanged between 2.5 and 10 vol% WC, while the fracture toughness decreases. Compared to Mo2C, the lower fracture toughness of NbC-Ni-WC cermets is attributed to the poorer wettability with the Ni binder.
3.4 NbC-Fe cermets
Due to its low solubility in iron, NbC in NbC-Fe cermets is advantageous for use as cutting tool [4], because it reduces diffusion between cutting tool and workpiece during machining and thus increases the cutting time.
3.4.1 Iron and Fe3Al binder
Esteban et al. [50] investigated the microstructural development and hardness of NbC-30Fe (wt%) cermets. To reduce particle size and improve the powder microstructure, they either used comminution milling or mechanical milling. The powders are compacted using either uniaxial or cold isostatic pressing followed by sintering in vacuum. Compared to comminution milling, mechanical milling leads to a more significant improvement of mechanical properties. This is attributed to the higher energy stored within the material, which leads the liquid phase appear at temperatures lower than 1400 °C resulting in a more homogenous microstructure and less porosity. Furthermore, finer carbides improve the hardness compared to the comminution milled cermets. Esteban et al. also find a Fe2Nb intermetallic phase, which is more pronounced in the mechanical milled cermets and hardens the material further.
Huang et al. [46] investigated the effect of different binders on the NbC grain growth during pressureless LPS. The iron (Fe) binder shows well-faceted NbC grains with slight rounded edges, while small grains are almost spherical. In contrast, the NbC-Ni cermet shows angular NbC grains. The addition of 25 at% Al to the Fe binder leads to a significant NbC grain refinement of 5–10 µm, which is associated with a higher hardness, but lower fracture toughness. Franco et al. [51] also studied the influence of 5 and 10 wt% Al in NbC-Fe cermets produced by conventional sintering or field assisted hot pressing (FAHP). Due to the higher Al content in NbC-Fe-10Al, Al0.7Fe3Si0.3 has formed within the matrix contributing to corrosion, wear, and mechanical resistance. The Si content in NbC-Fe-5Al, which is with 8.2 wt% more than twice that of NbC-Fe-10Al, further leads to an increase in hardness. In another study, Franco et al. [52] find that the addition of carbon to the binder do not improve processing, microstructure and properties, instead complex carbides and oxicarbides have formed, which lowers the densification and hardness.
Fe3Al is an economic binder for NbC cermets and a good low-cost alternative to stainless steel. It has a higher ductility, lower density, and a very good oxidation resistance up to 1200 °C [4] compared to pure Fe. Furthermore, Fe3Al undergoes no phase transitions, except order-disorder transformations [53], which is advantageous in high temperature applications.
3.4.2 Steel binder
In 2016, Huang et al. [54] investigated NbC cermets bonded by 15 and 30 vol% 316 L or 430 L stainless steel and produced using LPS. The advantage of stainless steel is its high corrosion resistance and high tensile properties. Relative densities of 98.9 and 98.4% are achieved for NbC-15 vol% 316 L and NbC-15 vol% 430 L cermets, respectively. Both cermets consist of cubic NbC with a ferritic (bcc) and austenitic (fcc) steel binder. The cermets bonded with 316 L binder has much higher fraction of austenite than ferrite and vice versa for the 430 L binder. This was attributed to elements such as Cr, Mo, Si and Nb promoting ferrite, while Ni, Mn, C and N promote ferrite. Cermets with 15 vol% binder consist of mainly spherical small grains and coarse NbC grains with well-faceted boundaries and slightly rounded edges. This indicates a solution-reprecipitation grain growth mode, which is controlled by the diffusion of Nb and C in the liquid binder. In cermets with 30 vol% binder, the NbC grain contact is reduced, which prevents the coalescence of carbide grains. Consequently, more spherical shaped grains occur, due to a diffusion driven process and a narrower grain size distribution is observed. Wavelength dispersive spectrometry (WDS) shows Cr-rich grains, maybe M7C3 or M23C6 carbides, which might decrease the corrosion resistance of the NbC cermets. Therefore, 1.5 vol% TiH2 are added, which results in mainly round-shaped NbC grains and homogeneously distributed Cr in the well dispersed steel binder. Thermally annealing in vacuum and subsequently quenching in water also impeded the formation of Cr-rich carbides. The 430 L binder shows higher hardness than 316 L binder, due to the higher amount of the ferritic phase. The hardness decreases with increasing binder content, whereas the fracture toughness increases. This is attributed to the better shielding of the stress field in front of a crack tip with higher binder content. However, transgranular fracture is dominant, which indicates strong NbC-steel interface.
The effect of sintering temperature on the microstructure of NbC cermets bonded by M48 high speed tool steel (HSS) was investigated by Hadian et al. [55]. Compared to the study of Huang et al., better wettability and sintering behavior is expected due to the presence of Co in the M48 HSS. Due to the low solubility of NbC in iron at low temperatures, an undistributed binder phase and thus higher porosity is expected. Above 1340 °C, the microstructure shows a homogeneous distribution of binder. NbC grain growth increases with increasing temperature. In addition, a uniform distribution of Co is reported within the microstructure, while Fe is mostly located in the binder phase. A sharp increase in hardness is observed from 1260 to 1300 °C, followed by a slight decrease with increasing sintering temperature. The broad deviation of the fracture resistance is attributed to the undistributed brittle eutectic carbides, which are observed in the HSS binder. Further, the increase in fracture resistance is mainly due to the NbC grain growth.
3.5 NbC-CoCrFeNiMn cermets
The high-entropy alloy (HEA) CoCrFeNiMn has very good mechanical properties, which makes it interesting for use as binder in NbC cermets. Shao et al. [56] produced NbC-CoCrFeNiMn cermets with a NbC content of 70 vol%. For reference, NbC-Ni cermets are also produced. Densities about 90% are achieved, which may relate to the poor wettability between NbC and the CoCrFeNiMn HEA binder. In comparison, NbC particles coarsen more in Ni binder than in CoCrFeNiMn binder. A small amount of Nb dissolves in the CoCrFeNiMn binder, while the Cr and Mn content are lower. The researchers explain this finding with the dissolution of Cr in NbC and the volatilization of Mn during sintering at high temperatures. In addition to the NbC grains and the CoCrFeNiMn binder, a Cr-rich phase occurs near the NbC grains with increasing sintering temperature. SEM-EDS investigations reveals a M23C6-type carbide (M = Cr, Co, Fe, Ni, Mn). The hardness and transverse rupture strength (TRS) decrease with increasing temperature, which is attributed to the larger particle size and the presence of the brittle M23C6 phase.
4 Tribological behavior
Since friction and wear play a major role in the application of cemented carbides, the tribological properties are an important basis for the use of a material. The wear rate kv is defined as the ratio of volumetric wear to the product of load FN and the sliding distance s. The total wear rate ktotal is thereby the sum of the wear rate of both bodies. The coefficient of friction f describes the resistance to motion due to friction and is the ratio between friction force and normal force.
Huang et al. investigated the oscillation wear behavior of pure NbC under three relative humidities φ = 2, 50 and 98% and showed that kv and f were almost independent from φ [42]. In a further study, Woydt and Mohrbacher stated that to estimate the tribological performance of the materials over a wide range of operating conditions, only one test was performed per parameter combination. Therefore, these results are only indicative and not statistically proven and therefore there might be no dependency on relative humidity for pure NbC. The wear rate of NbC is almost independent of the sliding speed and the wear resistance is one of the highest at a low sliding speeds compared to Cr3C2-NiCr, TiCxNy based cermets and WC cemented carbides [31]. The coefficient of friction increases with increasing sliding speed at room temperature, whereas the opposite occurs at 400 °C [28]. Woydt et al. also reported that during dry sliding, binderless NbC is comparable to other WC-based hard metals [28], which is the reason why NbC is mainly qualified for traction and frictional applications.
Woydt and Mohrbacher [33] also investigated the tribological behavior of NbC-Co cermets. The total wear rate ktotal versus the coefficient of friction f are shown in Fig. 12 at the end of the chapter. In a later study, Woydt and Mohrbacher [53] compared the tribological behavior of different NbC cermets, which is also presented in Fig. 12 in order to summarize the results. With increasing size of the markings, the relative humidity increases from 2, 50 to 98%, showing sensitivity to relative humidity for NbC-Co cermets. With increasing relative humidity, the coefficient of friction decreases. In case of HP-NbC, no statement on sensitivity can be made based on the data. The wear rates of HP-NbC and the NbC-Co cermets are quite similar and remain unchanged. Therefore, no improvement in tribological behavior can be observed during oscillating wear tests of NbC-Co cermets.
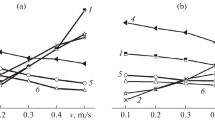
The aim of the use of iron based MMCs is to improve the wear resistance under dry sliding and the abrasive wear occurring in closed and opened tribological systems [57]. Wear occurs during processing and can be detected through wear tracks. Fig. 10 shows the coefficient of friction versus the sliding velocity at temperatures of 22 °C and 400 °C under continuous dry sliding conditions. Woydt and Mohrbacher [57] reported, that at room temperature, the wear rate decreases with increasing sliding speed. However, the NbC-Fe3Al cermet has a lower coefficient of friction between sliding speeds of approximately 0.2 and 3 m/s. At a temperature of 400 °C, the opposite occurs, and the cermet shows overall higher friction values than the MMC. The wear tracks show no grain pull-out or dominant fragmentation of NbC grains, indicating a high wear resistance. The investigation of oscillating wear under relative humidity of Fe3Al-NbC cermets showed that the coefficient of friction decreases with increasing relative humidity [10, 46]. This effect is dominated by the tribo-oxidative formation of Fe2O3 and/or by the hydrolysis to α‑, β‑ or γ‑FeOOH and Fe(OH)2 [42].
Coefficient of friction versus sliding speed at temperatures of a T = 22 °C and b T = 400 °C under continuously dry sliding of binderless NbC, NbC-Co cermets and a Fe3Al-based NbC cermet. (Adapted from [33])
Compared to pure NbC, Ni-based NbC cermets show a higher coefficient of friction at low sliding speeds at room temperature, as shown in Fig. 11. With increasing sliding speeds, f of NbC-Ni cermets decreases and is comparable to WC-6Ni at v = 3 m/s. Above this value at room temperature, and at T = 400 °C the NbC-Ni cermets show significantly improved tribological behavior compared to WC-6Ni and pure NbC. Woydt further reported that although Ni and NiMo-bonded NbC cermets exhibit lower toughness, they show a higher abrasive wear resistance and 30% to 100% higher tool life under dry turning compared to fine grained WC-6Co [4].
Coefficient of friction versus sliding speed at temperatures of a T = 22 °C and b T = 400 °C under continuously dry sliding of binderless NbC, and two NbC-Ni cermets. (Adapted from [58])
Fig. 12 shows ktotal of various hard metals and cermets under dry oscillation and different humidity levels versus Al2O3 or 100Cr6 as counterbody. With increasing markings, the relative humidity increases. Huang et al. [42] reported that the friction of NbC-Co cermets decreased less pronounced with increasing humidity than for NbC-Ni cermets, whereas the oscillating wear is independent from humidity for both types of cermet. Therefore, the type of binder affects the extend of sensitivity of NbC cermets to humidity. In case of Fe3Al, both f and ktotal decrease with increasing humidity, which is attributed to the tribo-oxidatively formation of Fe2O3 and/or the hydrolyzation to α‑, β‑ or γ‑FEOOH and Fe(OH)2 [42].
Total wear rate versus coefficient of friction of various hard metals and cermets under dry oscillation and different relativ humidity levels versus: a Al2O3; b 100Cr6. The increasing humidity is shown with increasing markings. (Adapted from [53])
Overall, the comparison with established cutting tools shows that the tribological behavior of all NbC cermets is comparable to WC and Cr3C2 hard metals or cermets under dry friction.
5 Conclusions and outlook
Extensive attention has been drawn on the production and investigation of NbC cermets. Between 2000 and 2014, studies intensively investigated NbC-Co cermets. NbC-Co shows similar hardness, but lower toughness than WC cemented carbides. Through the substitution of NbC1.0 to a sub-stoichiometric NbC0.88, hardness increased in NbC0.88-12Co, but the wear product Co3O4 is still a health issue. Therefore, Iron-bonded NbC cermets have recently been investigated. The ratio of hardness to fracture toughness, however, has been in most cases worse than that of NbC-Ni cermets. This is the reason why most of the research has focused on Nickel-bonded NbC cermets with different secondary carbides and contents. Different process technologies were used to produce the cermets. The focus was on powder metallurgical production: mechanical milling and the production of a colloid of the powder, pressing and sintering through hot pressing, SPS, LPS and further densification through GPS or HIP. There have also been successful attempts to cast Fe3Al-NbC metal matrix composites.
The resulting microstructure and mechanical properties are directly affected by the wetting behavior of the binder and the secondary carbides to the NbC grains. The content of binder and secondary carbides also plays an important role. The microstructure forms mainly through dissolution of the binder and secondary carbides in the NbC grains and the dissolution of NbC in the binder. Secondary carbides with a high formation enthalpy and therefore a good wetting behavior tend to completely dissolute to form solid solutions of the type (Nb, MeC)C. TiC and WC are known to form core-rim structures with NbC grains. On the one hand, the dissolution of secondary carbides is a problem in terms of hindering grain growth and the hardness cannot be effectively increased. On the other hand, the formation of solid solution carbides leads to a chemical interface bond, which guarantees stress transfer under load.
Besides the influence of binder and secondary carbides on phase formation, the sintering method affects the diffusion and thus the grain growth rate. While fast heating and cooling during SPS leads to a fine grain size, LPS leads to grain growth and thus to a decrease in hardness but increase in fracture toughness. However, SPS cannot currently be used in industry without restrictions. Since a certain fracture toughness is required for a high cutting time and machining performance, LPS is preferred in recent studies. Even though manufacturing processes and their influence on the microstructure have been intensively investigated, limited information is available about the tribological behavior and machining performance of NbC cermets. Hence, there is significant potential for research into this area to minimize wear and maximize service life. Not many researches have been conducted investigating the wear mechanisms. Uncoated NbC inserts achieved higher resistance to abrasive wear than coated WC inserts and a study of Uhlmann et al. [59] showed that coated NbC inserts are as good as coated WC inserts. Further studies should therefore focus on the influence of the microstructure and coatings on the wear behavior and machining performance.
References
Mari D, Llanes L, Nebel CE (2014) Comprehensive hard materials. Elsevier, Amsterdam
WHO-IARC (2006) Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. Monographs on the evaluation of carcinogenic risks to humans, vol 86, pp 1–294
Program NT (2014) Technical report 581 on the toxicology studies of cobalt metal. NIH publication (CAS NO. 7440-48-4)
Woydt M (2017) Status of nickel bonded niobium carbide (NbC) as a substitute for cobalt-bonded tungsten carbide (WC) as cutting tools and for wear protection. Powdermet 2017. Las Vegas, USA
European chemicals agency (ECHA) https://www.echa.europa.eu/information-on-chemicals. Accessed 29 July 2020
Shabalin I (2019) Ultra-High Temperature Materials II. Refractory Carbides I (Ta, Hf, Nb and Zr Carbides) vol 2. Springer Nature, Berlin https://doi.org/10.1007/978-94-024-1302-1_0
European Chemicals Agency (ECHA) (2020) Niobium carbide—Scientific properties. https://echa.europa.eu/de/brief-profile/-/briefprofile/100.031.913. Accessed 29 July 2020
Pierson HO (1996) Handbook of refractory carbides & nitrides: Properties, characteristics, processing and applications. William Andrew, Norwich
Kurlov A, Gusev A (2006) Tungsten carbides and W‑C phase diagram. Inorg Mater 42:121–127. https://doi.org/10.1134/S0020168506020051
Huang SG, Vanmeensel K, Mohrbacher H, Woydt M, Vleugels J (2015) Microstructure and mechanical porperties of NbC-matrix hardmetals with secondary carbide addition and different metal binders. Int J Refract Hard Met 48:418–426
Warren R (1968) Microstructural development during the liquid-phase sintering of two-phase alloys, with special reference to the NbC/Co system. J Mater Sci 3(5):471–485. https://doi.org/10.1007/BF00549730
Warren R (1969) Carbide grain growth during the liquid-phase sintering of the alloys NbC·Fe, NbC·Ni, and NbC·Co. J Less Common Met 17(1):65–72. https://doi.org/10.1016/0022-5088(69)90037-X
Lafreniere S, Irons GA (1990) Sedimentation during liquid processing of metal matrix composites. In: Bouchard M, Tremblay P (eds) Production, refining, fabrication and recycling of light metals. Pergamon, Oxford, pp 177–186 https://doi.org/10.1016/B978-0-08-040416-5.50021-7
Terry BS, Chinyamakobvu OS (1992) Dispersion and reaction of TiC in liquid iron alloys. Mater Sci Technol 8(5):399–405. https://doi.org/10.1179/mst.1992.8.5.399
Moon H, Kim B‑K, Kang LS‑J (2001) Growth mechanism of round-edged NbC grains in Co liquid. Acta Mater 49(7):1293–1299. https://doi.org/10.1016/S1359-6454(00)00394-3
Huang SG, Li L, Van der Biest O, Vleugels J (2007) Influence of WC addition on the microstructure and mechanical properties of NbC–Co cermets. J Alloys Compd 430(1):158–164. https://doi.org/10.1016/j.jallcom.2006.05.015
Huang SG, Van der Biest O, Li L, Vleugels J (2007) Properties of NbC–Co cermets obtained by spark plasma sintering. Mater Lett 61(2):574–577. https://doi.org/10.1016/j.matlet.2006.05.011
Cuppari MGD, Santos SF (2016) Physical properties of the NbC carbide. Metals 6(10):17. https://doi.org/10.3390/met6100250
Woydt M, Mohrbacher H (2015) The use of niobium carbide (NbC) as cutting tools and for wear resistant tribosystems. Int J Refract Hard Met 49:212–218. https://doi.org/10.1016/j.ijrmhm.2014.07.002
Nedfors N, Tengstrand O, Lewin E, Furlan A, Eklund P, Hultman L, Jansson U (2011) Structural, mechanical and electrical-contact properties of nanocrystalline-NbC/amorphous‑C coatings deposited by magnetron sputtering. Surf Coat Technol 206(2):354–359. https://doi.org/10.1016/j.surfcoat.2011.07.021
Williams WS (1998) The thermal conductivity of metallic ceramics. JOM 50(6):62–66. https://doi.org/10.1007/s11837-998-0131-y
Zhong Y, Xia X, Shi F, Zhan J, Tu J, Fan HJ (2016) Transition metal carbides and nitrides in energy storage and conversion. Adv Sci 3(5):1500286. https://doi.org/10.1002/advs.201500286
Genga RM, Cornish LA, Woydt M, Janse van Vuuren A, Polese C (2018) Microstructure, mechanical and machining properties of LPS and SPS NbC cemented carbides for face-milling of grey cast iron. Int J Refract Hard Met 73:111–120. https://doi.org/10.1016/j.ijrmhm.2017.12.036
Genga RM, Cornish LA, Akdogan G (2013) Effect of Mo2C additions on the properties of SPS manufactured WC–TiC–Ni cemented carbides. Int J Refract Hard Met 41:12–21. https://doi.org/10.1016/j.ijrmhm.2013.01.008
Genga RM, Akdogan G, Polese C, Garrett JC, Cornish LA (2015) Abrasion wear, thermal shock and impact resistance of WC-cemented carbides produced by PECS and LPS. Int J Refract Hard Met 49:133–142. https://doi.org/10.1016/j.ijrmhm.2014.07.031
Wäsche R (2017) Colloidal processing of metal bonded niobium carbide (NbC-Ni). 19. Plansee Seminar. Reutte, Austria
Genga RM, Cornish LA, Woydt M, Sobiyi K, Polese C (2018) Wear and mechanical properties of spark plasma and liquid phase sintered WC and NbC based cemented carbide inserts. In: Proceedings of the International Symposium on Wear Resistant Alloy for the Mining and Processing Industry, Sao Paulo, Brazil, 04.05.2015, pp 547–567
Woydt M, Mohrbacher H (2013) Friction and wear of binder-less niobium carbide. Wear 306(1):126–130. https://doi.org/10.1016/j.wear.2013.07.013
Song S‑X, Wang Z, Shi G‑P (2013) Heating mechanism of spark plasma sintering. Ceram Int 39(2):1393–1396. https://doi.org/10.1016/j.ceramint.2012.07.080
Genga RM, Rokebrand P, Cornish LA, Zeman P, Brajer J, Woydt M, van Vuuren AJ, Polese C (2020) Roughing, semi-finishing and finishing of laser surface modified nickel bonded NbC and WC inserts for grey cast iron (GCI) face-milling. Int J Refract Hard Met 86:105128. https://doi.org/10.1016/j.ijrmhm.2019.105128
Woydt M (2014) Mohrbacher H The use of niobium carbide (NbC) for wear resistant tribosystems and the tribological backgrounds as cutting tools. In: 9th International conference on tungsten, refractory & hardmaterials (Proceedings), Orlando, FL, USA, 18.05.2014, pp 222–232
Cho YK, Yoon DY, Kim B‑K (2004) Surface roughening transition and coarsening of NbC grains in liquid cobalt-rich matrix. J Am Ceram Soc 87(3):443–448. https://doi.org/10.1111/j.1551-2916.2004.00443.x
Woydt M, Mohrbacher H (2014) The tribological and mechanical properties of niobium carbides (NbC) bonded with cobalt or Fe3Al. Wear 321:1–7. https://doi.org/10.1016/j.wear.2014.09.007
Woydt M, Mohrbacher H, Vleugels J, Huang S (2017) Tailoring the functional properties of niobium carbide. In: Advanced processing and manufacturing technologies for nanostructured and multifunctional materials III, pp 101–113 https://doi.org/10.1002/9781119321736.ch11
Huang SG, Vleugels J, Altintas BT, Huang JH, Lauwers B, Qian J (2021) Microstructure and mechanical properties of WC and Ti(C0.7N0.3) modified NbC solid solution cermets. J Alloys Compd 850:156594. https://doi.org/10.1016/j.jallcom.2020.156594
Woydt M, Mohrbacher H (2015) The background for the use of hardmetals and MMCs based on niobium carbide (NbC) as cutting tools and for wear resistant tribosystems. In: Proceedings of the 3rd international conference on stone and concrete machining (ICSCM), Bochum, Germany, 02.11.2015, pp 199–207
Ramqvist L (1965) Wetting of metallic carbides by liquid copper, nickel, cobalt and iron. Int J Powder Met 1(4):2–21
Huang SG, Vleugels J, Mohrbacher H, Woydt M (2018) NbC grain growth control and mechanical properties of Ni bonded NbC cermets prepared by vacuum liquid phase sintering. Int J Refract Hard Met 72:63–70. https://doi.org/10.1016/j.ijrmhm.2017.12.013
Huang S, De Beats P, Sukumaran J, Mohrbacher H, Woydt M, Vleugels J (2018) Effect of carbon content on the microstructure and mechanical properties of NbC-Ni based cermets. Metals 8(3):171–113. https://doi.org/10.3390/met8030178 (Article 178)
Huang SG, Vleugels J, Mohrbacher H, Woydt M (2016) Microstructure and mechanical properties of NbC matrix cermets using Ni containing metal binder. Met Powder Rep 71(5):349–355
Barranco J, Warenchak R (1989) Liquid phase sintering of carbides using a nickel-molybdenum alloy. Int J Refract Hard Met 8(2):102–110
Huang SG, Vleugels J, Mohrbacher H, Woydt M (2017) Microstructure and tribological performance of NbC-Ni cermets modified by VC and Mo2C. Int J Refract Hard Met 66:188–197. https://doi.org/10.1016/j.ijrmhm.2017.03.012
Kayser GF (1989) The lattice parameters and microstructures of annealed, nickel-rich nickel-molybdenum alloys. J Mater Sci 24(8):2677–2680. https://doi.org/10.1007/BF02385610
Labonne M, Missiaen J‑M, Lay S, García N, Lavigne O, García LF, Tarrés E (2020) Sintering behavior and microstructural evolution of NbC-Ni cemented carbides with Mo2C additions. Int J Refract Hard Met 92:105295. https://doi.org/10.1016/j.ijrmhm.2020.105295
Huang JH, Huang SG, Zhou P, Lauwers B, Qian J, Vleugels J (2020) Microstructure and mechanical properties of WC or Mo2C modified NbC-Ni cermets. Int J Refract Hard Met. https://doi.org/10.1016/j.ijrmhm.2020.105440
Huang SG, Vleugels J, Mohrbacher H, Woydt M (2018) NbC-based cermets: influence of secondary carbide addition and metal binder. In: Proceedings of the International Symposium on Wear Resistant Alloy for the Mining and Processing Industry, Sao Paulo, Brazil, 04.05.2015, pp 521–534
Huang SG, Vleugels J, Mohrbacher H, Woydt M (2018) Effect of Ti(C0.7N0.3) content on the microstructure and mechanical properties of Ni bonded NbC-Ti(C0.7N0.3) based cermets. Solid State Phenom 274:43–52. https://doi.org/10.4028/www.scientific.net/SSP.274.43
Huang SG, Nie HB, Guo XY, Vleugels J, Huang JH, Mohrbacher H, Rajendhran NK, Sukumaran J, De Baets P, Cannizza E, Woydt M (2019) Microstructural investigation and machining performance of NbC-Ti(C0.5N0.5) matrix cermets. Int J Refract Hard Met 84:105038. https://doi.org/10.1016/j.ijrmhm.2019.105038
Ettmayer P, Kolaska H, Lengauer W, Dreyer K (1995) Ti(C,N) cermets—Metallurgy and properties. Int J Refract Hard Met 13(6):343–351. https://doi.org/10.1016/0263-4368(95)00027-G
Esteban PG, Gordo E (2006) Development of Fe–NbC cermet from powder obtained by self-propagating high temperature synthesis. Powder Met 49(2):153–159. https://doi.org/10.1179/174329006X102825
Franco E, Bonetti I, Tsipas SA, Gordo E, da Costa CE (2017) Processing and analysis of FeNbC cermets. Int J Refract Hard Met 62:29–36. https://doi.org/10.1016/j.ijrmhm.2016.10.012
Franco E, da Costa CE, Tsipas SA, Gordo E (2015) Cermets based on FeAl–NbC from composite powders: Design of composition and processing. Int J Refract Hard Met 48:324–332. https://doi.org/10.1016/j.ijrmhm.2014.09.030
Woydt M, Mohrbacher H (2018) The tribological property profile of hard metals and metal matrix composites based on niobium carbide. In: Proceedings of the International Symposium on Wear Resistant Alloys for the Mining and Processing Industry, Sao Paulo, Brazil, 04.05.2015, pp 427–461
Huang SG, Vleugels J, Mohrbacher H (2017) Stainless steel bonded NbC matrix cermets using a submicron NbC starting powder. Int J Refract Hard Met 63:26–31. https://doi.org/10.1016/j.ijrmhm.2016.04.021
Hadian A, Zamani C, Clemens FJ (2018) Effect of sintering temperature on microstructural evolution of M48 high speed tool steel bonded NbC matrix cemented carbides sintered in inert atmosphere. Int J Refract Hard Met 74:20–27. https://doi.org/10.1016/j.ijrmhm.2018.02.021
Shao Y, Guo Z, Wang Y, Ma H (2021) Fabrication and characterization of NbC-CoCrFeNiMn high-entropy alloy cermets. Int J Refract Hard Met 94:105388. https://doi.org/10.1016/j.ijrmhm.2020.105388
Woydt M, Mohrbacher H (2015) Hardmetals based on niobium carbide (NbC) versus casted NbC bearing MMCs. In: Singh D, Salem J, Wang J, Kirihara S (eds) 39th International conference on advanced ceramics and composites (Proceedings)—Mechanical properties and performance of engineering ceramics and composites X, Daytona Beach, FL, USA, 25.01.2015, pp 87–92
Woydt M, Huang S, Vleugels J, Mohrbacher H, Cannizza E (2018) Tailoring the functional profile of niobium carbide (NbC) as cutting tool materials and for wear protection. In: 2018 World Congress on Powder Metallurgy, Beijing, China, 16.09.2018, pp 785–795
Uhlmann E, Hinzmann D, Kropidlowksi K, Meier P, Prasol L, Woydt M (2018) Substitution of commercially coated tungsten carbide tools in dry cylindrical turning process by HiPIMS coated niobium carbide cutting inserts. Surf Coat Technol 354:112–118. https://doi.org/10.1016/j.surfcoat.2018.07.105
Acknowledgements
The authors thank Joachim Deinhofer for literature research.
Funding
The authors were funded in the project WO521/18‑3 by the Deutsche Forschungsgemeinschaft (DFG).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have received research grants from the Deutsche Forschungsgemeinschaft (DFG; WO521/18-3). D. Hübler and T. Gradt declare that they have no competing interests.
Additional information
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hübler, D., Gradt, T. Effect of different binders and secondary carbides on NbC cermets. Forsch Ingenieurwes 86, 197–211 (2022). https://doi.org/10.1007/s10010-022-00583-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10010-022-00583-1