Abstract
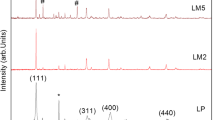
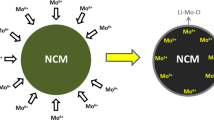
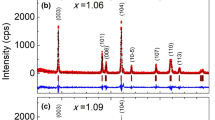
The Li[Li0.2Mn0.54Ni0.13Co0.13]O2 materials doped with different Mo content were studied using electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray analysis (EDX), and X-ray diffractometry (XRD). The electrochemical properties were also measured. An increase of Mo content resulted in a bigger lattice volume and a lower cation disorder. In addition, the electrochemical performance was enhanced with the increasing Mo content. However, more aggregation of particles was found to occur at a higher Mo content, which resulted in worse electrochemical performance. The highest electrochemical performance was obtained with a 5 mol% Mo addition.








Similar content being viewed by others
References
Jafta CJ, Ozoemena KI, Mathe MK, Roos WD (2012) Synthesis, characterisation and electrochemical intercalation kinetics of nanostructured aluminium-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium ion battery. Electrochim Acta 85:411–422
Singh G, West WC, Soler J, Katiyar RS (2012) In situ Raman spectroscopy of layered solid solution Li2MnO3–LiMO2 (M = Ni, Mn, Co). J Power Sources 218:34–38
West W, Soler J, Ratnakumar B (2012) Preparation of high quality layered-layered composite Li2MnO3−LiMO2(M = Ni, Mn, Co) Li-ion cathodes by a ball milling–annealing process. J Power Sources 204:200–204
Yang S, Huang G, Hu S, Hou X, Huang Y, Yue M, Lei G (2014) Improved electrochemical performance of the Li1.2Ni0.13Co0.13Mn0.54O2 wired by CNT networks for lithium-ion batteries. Mater Lett 118:8–11
Zhao C, Wang X, Liu X, Zhang H, Shen Q (2014) Mn–Ni content-dependent structures and electrochemical behaviors of serial Li1.2Ni0.13 + x Co0.13Mn0.54 − x O2 as lithium-ion battery cathodes. ACS Appl Mater Interfaces 6:2386–2392
Li Q, Li G, Fu C, Luo D, Fan J, Li L (2014) K+-doped Li1.2Mn0.54Co0.13Ni0.13O2: a novel cathode material with an enhanced cycling stability for lithium-ion batteries. ACS Appl Mater Interfaces 6:10330–10341
Song B, Liu H, Liu Z, Xiao P, Lai MO, Lu L (2013) High rate capability caused by surface cubic spinels in Li-rich layer-structured cathodes for Li-ion batteries. Sci Rep 3:3094
Zheng J, Wu X, YANG Y (2011) A comparison of preparation method on the electrochemical performance of cathode material Li [Li0.2Mn0.54Ni0.13Co0.13]O2 for lithium ion battery. Electrochim Acta 56:3071–3078
Gao J, Kim J, Manthiram A (2009) High capacity Li [Li0.2Mn0.54Ni0.13Co0.13]O2–V2O5 composite cathodes with low irreversible capacity loss for lithium ion batteries. Electrochem Commun 11:84–86
Lee E-S, Manthiram A (2014) Smart design of lithium-rich layered oxide cathode compositions with suppressed voltage decay. J Mater Chem 2:3932–3939
Cong L-N, Gao X-G, Ma S-C, Guo X, Zeng Y-P, Tai L-H, Wang R-S, Xie H-M et al (2014) Enhancement of electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by surface modification with Li4Ti5O12. Electrochim Acta 115:399–406
Wu F, Wang Z, Su Y, Yan N, Bao L, Chen S (2014) Li[Li0.2Mn0.54Ni0.13Co0.13]O2–MoO3 composite cathodes with low irreversible capacity loss for lithium ion batteries. J Power Sources 247:20–25
Li L, Zhang X, Chen R, Zhao T, Lu J, Wu F, Amine K (2014) Synthesis and electrochemical performance of cathode material Li1.2Co0.13Ni0.13Mn0.54O2 from spent lithium-ion batteries. J Power Sources 249:28–34
Whitfield P, Davidson I, Cranswick L, Swainson I, Stephens P (2005) Investigation of possible superstructure and cation disorder in the lithium battery cathode material LiMn< sub> 1/3</sub> Ni< sub> 1/3</sub> Co< sub> 1/3</sub> O< sub> 2</sub> using neutron and anomalous dispersion powder diffraction. Solid State Ionics 176:463–471
Zheng J, Wu X, Yang Y (2013) Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material by fluorine incorporation. Electrochim Acta 105:200–208
Du K, Yang F, G-r H, Z-d P, Y-b C, Ryu KS (2013) Sodium additive to improve rate performance of Li Li0.2Mn0.54Ni0.13Co0.13 O2 material for Li-ion batteries. J Power Sources 244:29–34
Park J-H, Lim J, Yoon J, Park K-S, Gim J, Song J, Park H, Im D et al (2012) The effects of Mo doping on 0.3Li[Li0.33Mn0.67]O2·0.7Li[Ni0.5Co0.2Mn0.3]O2 cathode material. Dalton Trans 41:3053–3059
Takahashi Y, Kijima N, Hayakawa H, Awaka J, Akimoto J (2008) Single-crystal synthesis and structure refinement of Li2MoO3. J Phys Chem Solids 69:1518–1520
Wu F, Tian J, Su Y, Guan Y, Jin Y, Wang Z, He T, Bao L et al (2014) Lithium-active molybdenum trioxide coated LiNi0.5Co0.2Mn0.3O2 cathode material with enhanced electrochemical properties for lithium-ion batteries. J Power Sources 269:747–754
Gao Y, Ma J, Wang X, Lu X, Bai Y, Wang Z, Chen L (2014) Improved electron/Li-ion transport and oxygen stability of Mo-doped Li2MnO3. J Mater Chem 2:4811–4818
Konishi H, Yoshikawa M, Hirano T (2013) The effect of thermal stability for high-Ni-content layer-structured cathode materials, LiNi0.8Mn0.1 − x Co0.1Mo x O2 (x = 0, 0.02, 0.04). J Power Sources 244:23–28
Jiang K-C, Xin S, Lee J-S, Kim J, Xiao X-L, Guo Y-G (2012) Improved kinetics of LiNi1/3Mn1/3Co1/3O2 cathode material through reduced graphene oxide networks. Phys Chem Chem Phys 14:2934–2939
Wang J, Yao XY, Zhou XF, Liu ZP (2011) Synthesis and electrochemical properties of layered lithium transition metal oxides. J Mater Chem 21:2544–2549
Bai Y, Wang X, Zhang X, Shu H, Yang X, Hu B, Wei Q, Wu H et al (2013) The kinetics of Li-ion deintercalation in the Li-rich layered Li1.12[Ni0.5Co0.2Mn0.3]0.89O2 studied by electrochemical impedance spectroscopy and galvanostatic intermittent titration technique. Electrochim Acta 109:355–364
Liu JL, Chen L, Hou MY, Wang F, Che RC, Xia YY (2012) General synthesis of xLi2MnO3·1 − x LiMn1/3Ni1/3Co1/3O2 nanomaterials by a molten-salt method: towards a high capacity and high power cathode for rechargeable lithium batteries. J Mater Chem 22:25380–25387
Zhao TL, Chen S, Li L, Zhang XF, Chen RJ, Belharouak I, Wu F, Amine K (2013) Synthesis, characterization, and electrochemistry of cathode material Li[Li0.2Co0.13Ni0.13Mn0.54]O2 using organic chelating agents for lithium-ion batteries. J Power Sources 228:206–213
Wang Q, Huo J, Zheng Y, Pang S, He Z (2013) Design of red/green emissive lanthanide activated nano-materials by supersonic and microwave co-irradiations. Opt Mater 35:1146–1150
Cho T-H, Shiosaki Y, Noguchi H (2006) Preparation and characterization of layered LiMn1/3Ni1/3Co1/3O2 as a cathode material by an oxalate co-precipitation method. J Power Sources 159:1322–1327
Xiao J, Chernova NA, Whittingham MS (2008) Layered mixed transition metal oxide cathodes with reduced cobalt content for lithium ion batteries. Chem Mater 20:7454–7464
Dorset DL, Siskin M (2008) Molecular assemblies in asphaltenes and their high-temperature coke products. Part 1: initial molecular organization. Energy Fuels 22:2512–2517
Yu S-H, Yoon T, Mun J, Park S, Kang Y-S, Park J-H, Oh SM, Sung Y-E (2013) Continuous activation of Li2MnO3 component upon cycling in Li1.167Ni0.233Co0.100Mn0.467Mo0.033O2 cathode material for lithium ion batteries. J Mater Chem 1:2833–2839
Johnson CS, Li N, Lefief C, Vaughey JT, Thackeray MM (2008) Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3 · (1 − x)LiMn0.333Ni0.333Co0.333O2 (0 ≤ x ≤ 0.7). Chem Mater 20:6095–6106
Moorhead-Rosenberg Z, Chemelewski KR, Goodenough JB, Manthiram A (2013) Magnetic measurements as a viable tool to assess the relative degrees of cation ordering and Mn3+ content in doped LiMn1.5Ni0.5O4 spinel cathodes. J Mater Chem 1:10745–10752
Liu W, Fang G, Xia B, Sun H, Kaneko S, Li D (2013) Improved electrochemical properties of Li[Li0.2Ni0.17Mn0.56Co0.07]O2 cathode material via micro-structural rearrangement. RSC Adv 3:15630–15635
Uzun D, Doğrusöz M, Mazman M, Biçer E, Avci E, Şener T, Kaypmaz TC, Demir-Cakan R (2013) Effect of MnO2 coating on layered Li(Li0.1Ni0.3Mn0.5Fe0.1)O2 cathode material for Li-ion batteries. Solid State Ionics 249–250:171–176
Wang GG, Wang JM, Mao WQ, Shao HB, Zhang JQ, Cao CN (2005) Physical properties and electrochemical performance of LiMn2O4 cathode materials prepared by a precipitation method. J Solid State Electrochem 9:524–530
Lee J, Kumar P, Moudgil BM, Singh RK (2013) Electrochemical enhancement of LiFePO4 as a cathode material by incorporating Cu flakes for lithium ion rechargeable battery. Solid State Ionics 231:18–24
Zhang QY, Zhang CL, Li B, Jiang DD, Kang SF, Li X, Wang YG (2013) Preparation and characterization of W-doped Li4Ti5O12 anode material for enhancing the high rate performance. Electrochim Acta 107:139–146
Acknowledgments
We are grateful for the financial support of this research work by the International Cooperation Program with Germany (2012DFG61480), the National High Technology Research and Development Program of China (2013AA050901).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, J., Shan, Z., Zhu, K. et al. Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by doping with molybdenum for Lithium battery. J Solid State Electrochem 19, 1037–1044 (2015). https://doi.org/10.1007/s10008-014-2706-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2706-6