Abstract
Context
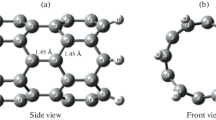
Nanosensor materials for the trapping and sensing of CO2 gas in the ecosystem were investigated herein to elucidate the adsorption, sensibility, selectivity, conductivity, and reactivity of silicon-doped carbon quantum dot (Si@CQD) decorated with Ag, Au, and Cu metals. The gas was studied in two configurations on its O and C sites. When the metal-decorated Si@CQD interacted with the CO2 gas on the C adsorption site of the gas, there was a decrease in all the interactions with the lowest energy gap of 1.084 eV observed in CO2_C_Cu_Si@CQD followed by CO2_C_Au_Si@CQD which recorded a slightly higher energy gap of 1.094 eV, while CO2_C_Ag_Si@CQD had an energy gap of 2.109 eV. On the O adsorption sites, a decrease was observed in CO2_O_Au_Si@CQD which had the least energy gap of 1.140 eV, whereas there was a significant increase after adsorption in CO2_O_Ag_Si@CQD and CO2_O_Cu_Si@CQD with calculated ∆E values of 2.942 eV and 3.015 eV respectively. The adsorption energy alongside the basis set supposition error (BSSE) estimation reveals that CO2_C_Au_Si@CQD, CO2_C_Ag_Si@CQD, and CO2_C_Cu_Si@CQD were weakly adsorbed, while chemisorption was present in the CO2_O_Ag_Si@CQD, CO2_O_Cu_Si@CQD, and CO2_O_Au_Si@CQD interactions. Indeed, the adsorption of CO2 on the different metal-decorated quantum dots affects the Fermi level (Ef) and the work function (Φ) of each of the decorated carbon quantum dots owed to their low Ef values and high ∆Φ% which shows that they can be a prospective work function–based sensor material.
Methods
Electronic structure theory method based on first-principle density functional theory (DFT) computation at the B3LYP-GD3(BJ)/Def2-SVP level of theory was utilized through the use of the Gaussian 16 and GaussView 6.0.16 software packages. Post-processing computational code such as multi-wavefunction was employed for result analysis and visualization.








Similar content being viewed by others
References
Urban JJ, Talapin DV, Shevchenko EV, Murray CB (2006) Self-assembly of PbTe quantum dots into nanocrystal superlattices and glassy films. J Am Chem Soc 128(10):3248–3255
Jorns M, Pappas D (2021) A review of fluorescent carbon dots, their synthesis, physical and chemical characteristics, and applications. Nanomater 11(6):1448
Wang J, Liu G, Cham-Fai Leung K, Loffroy R, Lu PX, Wang XJ (2015) Opportunities and challenges of fluorescent carbon dots in translational optical imaging. Curr Pharm Des 21(37):5401–5416
Zhang X, Jiang M, Niu N, Chen Z, Li S, Liu S, Li J (2018) Natural-product-derived carbon dots: from natural products to functional materials. ChemSusChem 11(1):11–24
Heng ZW, Chong WC, Pang YL, Koo CH (2021) An overview of the recent advances of carbon quantum dots/metal oxides in the application of heterogeneous photocatalysis in photodegradation of pollutants towards visible-light and solar energy exploitation. J Environ Chem Eng 9(3):105199
Vyas Y, Chundawat P, Dharmendra D, Punjabi PB, Ameta C (2021) Review on hydrogen production photocatalytically using carbon quantum dots: future fuel. Int J Hydrog Energy 46(75):37208–37241
Gaur M, Misra C, Yadav AB, Swaroop S, Maolmhuaidh FÓ, Bechelany M, Barhoum A (2021) Biomedical applications of carbon nanomaterials: fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Mater 14(20):5978
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44(1):362–381
Henna TK, Pramod K (2020) Graphene quantum dots redefine nanobiomedicine. Mater Sci Eng C 110:110651
Yoo CS (2013) Physical and chemical transformations of highly compressed carbon dioxide at bond energies. Phys Chem Chem Phys 15(21):7949–7966
Ming T, de_ Richter R, Shen S, Caillol S (2016) Fighting global warming by greenhouse gas removal: destroying atmospheric nitrous oxide thanks to synergies between two breakthrough technologies. Environ Sci Pollut Res 23(7):6119–6138
Sovacool BK, Griffiths S, Kim J, Bazilian M (2021) Climate change and industrial F-gases: a critical and systematic review of developments, sociotechnical systems and policy options for reducing synthetic greenhouse gas emissions. Renew Sust Energ Rev 141:110759
Healy N, Stephens JC, Malin SA (2019) Embodied energy injustices: unveiling and politicizing the transboundary harms of fossil fuel extractivism and fossil fuel supply chains. Energy Res Soc Sci 48:219–234
Waldbusser GG, Hales B, Langdon CJ, Haley BA, Schrader P, Brunner EL et al (2015) Saturation-state sensitivity of marine bivalve larvae to ocean acidification. Nat Clim Chang 5(3):273–280
Lustig WP, Mukherjee S, Rudd ND, Desai AV, Li J, Ghosh SK (2017) Metal–organic frameworks: functional luminescent and photonic materials for sensing applications. Chem Soc Rev 46(11):3242–3285
Montaseri H, Forbes PB (2018) Analytical techniques for the determination of acetaminophen: a review. TrAC Trends Anal Chem 108:122–134
Dahmen J, Cook DJ, Wang X, Honglei W (2017) Smart secure homes: a survey of smart home technologies that sense, assess, and respond to security threats. J Reliab Intell Environ 3(2):83–98
Kaur N, Choudhary BC, Sharma RK (2021) Carbon-dioxide gas sensor using co-doped graphene nanoribbon: a first principle DFT study. Mater Today: Proc 45:5023–5028
Anisimov YA, Evitts RW, Cree DE, Wilson LD (2021) Polyaniline/biopolymer composite systems for humidity sensor applications: a review. Polym 13(16):2722
Srivastava M, Srivastava A (2019) Electron transport in CO2 adsorbed ZnO nanowire: DFT study. Chem Phys Lett 729:17–23
Adalikwu SA, Louis H, Iloanya AC, Edet HO, Akem MU, Eno EA, Manicum ALE (2022) B-and Al-doped porous 2D covalent organic frameworks as nanocarriers for biguanides and metformin drugs. ACS Applied Bio Materials 5(12):5887–5900
Wang R, Li G, Dong Y, Chi Y, Chen G (2013) Carbon quantum dot-functionalized aerogels for NO2 gas sensing. Anal Chem 85(17):8065–8069
Bhakat A, Pal A, Siddaramaiah R, Chattopadhyay A (2021) Complexation-based super crystalline assembly of zinc oxide quantum dots for sensitive carbon dioxide gas sensing. J Phys Chem C 125(22):12316–12323
He T, Shi Q, Wang H, Wen F, Chen T, Ouyang J, Lee C (2019) Beyond energy harvesting-multi-functional triboelectric nanosensors on a textile. Nano Energy 57:338–352
Hussain R, Saeed M, Mehboob MY, Khan SU, Khan MU, Adnan M et al (2020) Density functional theory study of palladium cluster adsorption on a graphene support. RSC Adv 10(35):20595–20607
Oishi AA, Dhali P, Das A, Mondal S, Rad AS, Hasan MM (2022) Study of the adsorption of chloropicrin on pure and Ga and Al doped B12N12: a comprehensive DFT and QTAIM investigation. Mol Simul 49:776–788
Shahabi M, Raissi H (2016) Molecular dynamics simulation and quantum chemical studies on the investigation of aluminum nitride nanotube as phosgene gas sensor. J Incl Phenom Macrocycl Chem 86(3):305–322
Venkataramanan NS, Ambigapathy S (2015) Encapsulation of sulfur, oxygen, and nitrogen mustards by cucurbiturils: a DFT study. J Incl Phenom Macrocycl Chem 83(3):387–400
An B, Feng S, Wen K, Wu W, Yuan H, Zhu Q et al (2017) Theoretical insights into the ultrafast excited-state intramolecular proton transfer (ESIPT) mechanism in a series of amide-based NH⋯ N hydrogen-bonding compounds. Org Electron 45:1–8
Agwupuye JA, Neji PA, Louis H, Odey JO, Unimuke TO, Bisiong EA et al (2021) Investigation on electronic structure, vibrational spectra, NBO analysis, and molecular docking studies of aflatoxins and selected emerging mycotoxins against wild-type androgen receptor. Heliyon 7(7):e07544
Mumit MA, Pal TK, Alam MA, Islam MAAAA, Paul S, Sheikh MC (2020) DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2, 4, 5-trimethoxyphenylmethylene) hydrazinecarbodithioate. J Mol Struct 1220:128715
Andrienko GA (2010) Chemcraft-graphical software for visualization of quantum chemistry computations. https://www.chemcraftprog.com
Moberly JG, Bernards MT, Waynant KV (2018) Key features and updates for origin 2018. J Cheminformatics 10(1):1–2
Mohammadi MD, Abdullah HY (2021) The adsorption of bromochlorodifluoromethane on pristine, Al, Ga, P, and As-doped boron nitride nanotubes: a study involving PBC-DFT, NBO analysis, and QTAIM. Comput Theor Chem 1193:113047
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Stone JE, Hynninen AP, Phillips JC, Schulten K (2016) Early experiences porting the NAMD and VMD molecular simulation and analysis software to GPU-accelerated OpenPOWER platforms. International conference on high performance computing. Springer, Cham, pp 188–206
Ullah Z, Sattar F, Kim HJ, Jang S, Mary YS, Zhan X, Kwon HW (2022) Computational study of toxic gas removal. J Mol Liq 365:120213
Kim E, Safavi-Naini A, Hite DA, McKay KS, Pappas DP, Weck PF, Sadeghpour HR (2017) Electric-field noise from carbon-adatom diffusion on a Au (110) surface: first-principles calculations and experiments. Phys Rev A 95(3):033407
Kruse H, Grimme S (2012) A geometrical correction for the inter-and intra-molecular basis set superposition error in Hartree-Fock and density functional theory calculations for large systems. J Chem Phys 136(15):04B613
Habibinejad M, Khordad R, Ghanbari A (2021) Specific heat at constant pressure, enthalpy and Gibbs free energy of boron nitride (BN) using q-deformed exponential-type potential. Phys B Condens Matter 613:412940
Yoosefian M, Etminan N (2016) The role of solvent polarity in the electronic properties, stability and reactivity trend of a tryptophane/Pd doped SWCNT novel nanobiosensor from polar protic to non-polar solvents. RSC Adv 6(69):64818–64825
Wagner JP, Schreiner PR (2015) London dispersion in molecular chemistry—reconsidering steric effects. Angew Chem Int Ed 54(42):12274–12296
Devasia J, Chinnam S, Khatana K, Shakya S, Joy F, Rudrapal M, Nizam A (2022) Synthesis, DFT and in silico anti-COVID evaluation of novel tetrazole analogues. Polycycl Aromat Compd 43:1–16
Mohammadi MD, Abbas F, Louis H, Afahanam LE, Gber TE (2022) Intermolecular interactions between nitrosourea and polyoxometalate compounds. ChemistrySelect 7(36):e202202535
Louis H, Etiese D, Unimuke TO, Owen AE, Rajee AO, Gber TE et al (2022) Computational design and molecular modeling of the interaction of nicotinic acid hydrazide nickel-based complexes with H 2 S gas. RSC Adv 12(47):30365–30380
Morales-Garcia A, Valero R, Illas F (2017) Performance of the G 0 W 0 method in predicting the electronic gap of TiO2 nanoparticles. J Chem Theory Comput 13(8):3746–3753
Jalali Sarvestani MR, Doroudi Z (2020) Fullerene (C20) as a potential sensor for thermal and electrochemical detection of amitriptyline: a DFT study. J Chem Lett 1(2):63–68
Hussain S, Guo L, Louis H, Zhu S, He T (2019) First-principles calculations of wurtzite ZnS1-xSex solid solutions for photocatalysis. Mater Today Commun 21:100672
Louis H, Ekereke EE, Isang BB, Ikeuba AI, Amodu IO, Gber TE et al (2022) Assessing the performance of Al12N12 and Al12P12 nanostructured materials for alkali metal ion (Li, Na, K) batteries. ACS omega 7(50):46183–46202 https://pubs.acs.org/doi/full/10.1021/acsomega.2c04319
John S, Joseph A, Sajini T, Jose AJ (2017) Corrosion inhibition properties of 1, 2, 4-hetrocyclic systems: electrochemical, theoretical and Monte Carlo simulation studies. Egypt J Pet 26(3):721–732
Choudhary V, Bhatt A, Dash D, Sharma N (2019) DFT calculations on molecular structures, HOMO–LUMO study, reactivity descriptors and spectral analyses of newly synthesized diorganotin (IV) 2-chloridophenylacetohydroxamate complexes. J Comput Chem 40(27):2354–2363
Obot IB, Kaya S, Kaya C, Tüzün B (2016) Density functional theory (DFT) modeling and Monte Carlo simulation assessment of inhibition performance of some carbohydrazide Schiff bases for steel corrosion. Physica E Low Dimens Syst Nanostruct 80:82–90
Chandra AK, Nguyen MT (2002) Use of local softness for the interpretation of reaction mechanisms. Int J Mol Sci 3(4):310–323
Morell C, Gázquez JL, Vela A, Guégan F, Chermette H (2014) Revisiting electroaccepting and electrodonating powers: proposals for local electrophilicity and local nucleophilicity descriptors. Phys Chem Chem Phys 16(48):26832–26842
Eno EA, Louis H, Unimuke TO, Gber TE, Mbonu IJ, Ndubisi CJ, Adalikwu SA (2022) Reactivity, stability, and thermodynamics of para-methylpyridinium-based ionic liquids: insight from DFT, NCI, and QTAIM. J Mol Liq 2(1):100030
Louis H, Isang BB, Unimuke TO, Gber TE, Amodu IO, Ikeuba AI, Adeyinka AS (2022) Modeling of Al12N12, Mg12O12, Ca12O12, and C23N nanostructured as potential anode materials for sodium-ion battery. J Solid State Electrochem 27:47–59
Hart JL, Hantanasirisakul K, Lang AC, Anasori B, Pinto D, Pivak Y et al (2019) Control of MXenes’ electronic properties through termination and intercalation. Nat Commun 10(1):1–10
Shokuhi Rad A, Esfahanian M, Maleki S, Gharati G (2016) Application of carbon nanostructures toward SO2 and SO3 adsorption: a comparison between pristine graphene and N-doped graphene by DFT calculations. J Sulphur Chem 37(2):176–188
Contreras P, Seijas L, Osorio D (2021) TDOS quantum mechanical visual analysis for single molecules. arXiv:2105.12830. https://doi.org/10.48550/arXiv.2105.12830
Louis H, Guo LJ, Zhu S, Hussain S, He T (2019) Computational study on interactions between CO2 and (TiO2) n clusters at specific sites. Chin J Chem Phys 32(6):674–686
Zhao L, Pan S, Holzmann N, Schwerdtfeger P, Frenking G (2019) Chemical bonding and bonding models of main-group compounds. Chem Rev 119(14):8781–8845
Al-Ahmary KM, Habeeb MM, Aljahdali SH (2019) Synthesis, spectroscopic studies and DFT/TD-DFT/PCM calculations of molecular structure, spectroscopic characterization and NBO of charge transfer complex between 5-amino-1, 3-dimethylpyrazole (5-ADMP) with chloranilic acid (CLA) in different solvents. J Mol Liq 277:453–470
Khalid M, Lodhi HM, Khan MU, Imran M (2021) Structural parameter-modulated nonlinear optical amplitude of acceptor–π–D–π–donor-configured pyrene derivatives: a DFT approach. RSC Adv 11(23):14237–14250
Mohammadi MD, Abdullah HY, Louis H, Mathias GE (2022) 2D boron nitride material as a sensor for H2SiCl2. Comput Theor Chem 1213:113742
Demircioğlu Z, Kaştaş ÇA, Büyükgüngör O (2015) Theoretical analysis (NBO, NPA, Mulliken population method) and molecular orbital studies (hardness, chemical potential, electrophilicity and Fukui function analysis) of (E)-2-((4-hydroxy-2-methylphenylimino) methyl)-3-methoxyphenol. J Mol Struct 1091:183–195
Enudi OC, Louis H, Edim MM, Agwupuye JA, Ekpen FO, Bisong EA, Utsu PM (2021) Understanding the aqueous chemistry of quinoline and the diazanaphthalenes: insight from DFT study. Heliyon 7(7):e07531
Apebende CG, Louis H, Owen AE, Benjamin I, Amodu IO, Gber TE, Asogwa FC (2022) Adsorption properties of metal functionalized fullerene (C59Au, C59Hf, C59Ag, and C59Ir) nanoclusters for application as a biosensor for hydroxyurea (HXU): insight from theoretical computation. Z Phys Chem 236(11-12):1515–1546
Kumar V, Bano A, Rajput K, Roy DR (2021) The interaction of two-dimensional P2SiS nanosheet with environmental toxic NCG molecules for sensor application: a DFT study. Sensors Actuators A Phys 322:112608
Al-Otaibi JS, Mary YS, Mary YS, Trivedi R, Chakrabory B, Thomas R (2022) Cluster formation between an oxadiazole derivative with metal nanoclusters (Ag/Au/Cu), graphene quantum dot sheets, SERS studies and solvent effects. Struct Chem 34:867–877
Philip M, Woldu AR, Akbar MB, Louis H, Cong H (2021) A facile synthesis of Cu catalysts with multiple high-index facets for the suppression of competing H 2 evolution during electrocatalytic CO 2 reduction. Nanoscale 13(5):3042–3048
Yang JP, Bussolotti F, Kera S, Ueno N (2017) Origin and role of gap states in organic semiconductor studied by UPS: as the nature of organic molecular crystals. J Phys D Appl Phys 50(42):423002
Louis H, Udoh EU, Amodu IO, Ekereke EE, Isang BB, Onyebuenyi IB, Adeyinka AS (2022) Modeling of Mg 12 O 11-X (X= B, N, P and S) Nanostructured materials as sensors for melamine (C 3 H 6 N 6). J Comput Biophys Chem 21(8):999–1021
Molina A, González J (2016) Pulse voltammetry in physical electrochemistry and electroanalysis. Monographs in Electrochemistry 25(1):161–177
Wang M, Tang C (2022) Silicon doped boron carbide (BC3) nanosheet as a promising sensitive material for detection of acetaminophen drug based on the DFT approach. Silicon 14(10):5463–5470
Liao Q, Mohr M, Zhang X, Zhang Z, Zhang Y, Fecht HJ (2013) Carbon fiber–ZnO nanowire hybrid structures for flexible and adaptable strain sensors. Nanoscale 5(24):12350–12355
Ogunwale GJ, Louis H, Gber TE, Adeyinka AS (2022) Modeling of pristine, Ir-and Au-decorated C60 fullerenes as sensors for detection of hydroxyurea and nitrosourea drugs. J Environ Chem Eng 10(6):108802
Saedi L, Maskanati M, Modheji M, Soleymanabadi H (2018) Tuning the field emission and electronic properties of silicon nanocones by Al and P doping: DFT studies. J Mol Graph Model 81:168–174
Mishra N, Pandey BP, Kumar S (2021) Impact of N 2 O gas adsorption upon electronic properties of 2D MoSe 2 monolayer: a DFT approach. IEEE Sensors J 21(8):9756–9762
Abelard J, Wilmsmeyer AR, Edwards AC, Gordon WO, Durke EM, Karwacki CJ et al (2016) Adsorption of substituted benzene derivatives on silica: effects of electron withdrawing and donating groups. J Phys Chem C 120(24):13024–13031
Louis H, Mathias GE, Ikenyirimba OJ, Unimuke TO, Etiese D, Adeyinka AS (2022) Metal-doped Al12N12X (X= Na, Mg, K) nanoclusters as nanosensors for carboplatin: insight from first-principles computation. J Phys Chem B 126(27):5066–5080
Fu Y, Rong M, Yang K, Yang A, Wang X, Gao Q et al (2016) Calculated rate constants of the chemical reactions involving the main byproducts SO2F, SOF2, SO2F2 of SF6 decomposition in power equipment. J Phys D Appl Phys 49(15):155502
Singh RN, Kumar A, Tiwari RK, Rawat P (2013) A combined experimental and theoretical (DFT and AIM) studies on synthesis, molecular structure, spectroscopic properties and multiple interactions analysis in a novel ethyl-4-[2-(thiocarbamoyl) hydrazinylidene]-3, 5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. Spectrochim Acta A Mol Biomol Spectrosc 112:182–190
Mohammadi MD, Abbas F, Louis H, Mathias GE, Unimuke TO (2022) Trapping of CO, CO2, H2S, NH3, NO, NO2, and SO2 by polyoxometalate compound. Comput Theor Chem 1215:113826
Noureddine O, Issaoui N, Gatfaoui S, Al-Dossary O, Marouani H (2021) Quantum chemical calculations, spectroscopic properties and molecular docking studies of a novel piperazine derivative. J King Saud Univ Sci 33(2):101283
Li H, Shu Y, Gao S, Chen L, Ma Q, Ju X (2013) Easy methods to study the smart energetic TNT/CL-20 co-crystal. J Mol Model 19(11):4909–4917
Inah BE, Louis H, Benjamin I, Unimuke TO, Adeyinka AS (2022) Computational study on the interactions of functionalized C24NC (NC= C,–OH,–NH2,–COOH, and B) with chloroethylphenylbutanoic acid. Can J Chem 101:11–24
Anithaa VS, Vijayakumar S, Sudha M, Shankar R (2017) Theoretical investigation on hydrogen bond interaction of diketo/keto-enol form uracil and thymine tautomers with intercalators. J Mol Model 23(12):1–16
Koumpouras K, Larsson JA (2020) Distinguishing between chemical bonding and physical binding using electron localization function (ELF). J Phys Condens Matter 32(31):315502
Louis H, Egemonye TC, Unimuke TO, Inah BE, Edet HO, Eno EA et al (2022) Detection of carbon, sulfur, and nitrogen dioxide pollutants with a 2D Ca12O12 nanostructured material. ACS omega 7(39):34929–34943
Wang H, Wang M, Liang X, Yuan J, Yang H, Wang S et al (2021) Organic molecular sieve membranes for chemical separations. Chem Soc Rev 50(9):5468–5516
Unimuke TO, Louis H, Eno EA, Agwamba EC, Adeyinka AS (2022) Meta-hybrid density functional theory prediction of the reactivity, stability, and IGM of azepane, oxepane, thiepane, and halogenated cycloheptane. ACS omega 7(16):13704–13720
Jadoon T, Mahmood T, Ayub K (2020) Silver-graphene quantum dots based electrochemical sensor for trinitrotoluene and p-nitrophenol. J Mol Liq 306:112878
Jadoon T, Carter-Fenk K, Siddique MBA, Herbert JM, Hussain R, Iqbal S et al (2020) Silver clusters tune up electronic properties of graphene nanoflakes: a comprehensive theoretical study. J Mol Liq 297:111902
Lv Z, Tek A, Da Silva F, Empereur-Mot C, Chavent M, Baaden M (2013) Game on, science-how video game technology may help biologists tackle visualization challenges. PLoS One 8(3):e57990
Valt M, Gaiardo A, Fabbri B, Spagnoli E, Caporali M, Malagù C et al (2022) Elucidating the ambient stability and gas sensing mechanism of nickel-decorated phosphorene for NO2 detection: a first-principles study. ACS omega 7(11):9808–9817
Oprea A, Degler D, Barsan N, Hemeryck A, Rebholz J (2019) Basics of semiconducting metal oxide–based gas sensors. Gas Sensors Based on Conducting Metal Oxides, pp 61–165
Louis H, Charlie DE, Amodu IO, Benjamin I, Gber TE, Agwamba EC, Adeyinka AS (2022) Probing the reactions of thiourea (CH4N2S) with metals (X= Au, Hf, Hg, Ir, Os, W, Pt, and Re) anchored on fullerene surfaces (C59X). ACS omega 7(39):35118–35135
Lorenz M, Civalleri B, Maschio L, Sgroi M, Pullini D (2014) Benchmarking dispersion and geometrical counterpoise corrections for cost-effective large-scale DFT calculations of water adsorption on graphene. J Comput Chem 35(24):1789–1800
Gber TE, Louis H, Owen AE, Etinwa BE, Benjamin I, Asogwa FC et al (2022) Heteroatoms (Si, B, N, and P) doped 2D monolayer MoS 2 for NH 3 gas detection. RSC Adv 12(40):25992–26010
Mishra A, Kumari U, Turlapati VY, Siddiqi H, Meikap BC (2020) Extensive thermogravimetric and thermo-kinetic study of waste motor oil based on iso-conversional methods. Energy Convers Manag 221:113194
Agwupuye JA, Louis H, Unimuke TO, David P, Ubana EI, Moshood YL (2021) Electronic structure investigation of the stability, reactivity, NBO analysis, thermodynamics, and the nature of the interactions in methyl-substituted imidazolium-based ionic liquids. J Mol Liq 337:116458
Marques JG, Costa AL, Pereira C (2019) Gibbs free energy (ΔG) analysis for the NaOH (sodium-oxygen-hydrogen) thermochemical water splitting cycle. Int J Hydrog Energy 44(29):14536–14549
O’Neill RT, Boulatov R (2021) The many flavours of mechanochemistry and its plausible conceptual underpinnings. Nat Rev Chem 5(3):148–167
Suzuki T, Sackmann A, Oprea A, Weimar U, Bârsan N (2020) Chemoresistive CO2 gas sensors based on La2O2CO3: sensing mechanism insights provided by operando characterization. ACS Sens 5(8):2555–2562
Acknowledgements
We appreciate the center for high-performance computing (CHPC), South Africa, for providing the computational resources needed to actualized this research.
Author information
Authors and Affiliations
Contributions
H.L: project conceptualization, design, resources, and supervision. G.O: writing, results extraction, analysis, and manuscript first draft: K.C: visualization. E.A: methodology: E.E: writing, review, and editing. A.A: resources, writing, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okon, G.A., Louis, H., Eno, E.A. et al. First-principle study of Cu-, Ag-, and Au-decorated Si-doped carbon quantum dots (Si@CQD) for CO2 gas sensing efficacies. J Mol Model 29, 229 (2023). https://doi.org/10.1007/s00894-023-05627-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05627-z