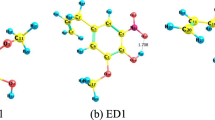
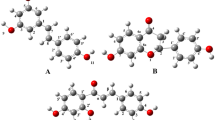
Abstract
Context
Some structural properties can be involved in the antioxidant capacity of several polyphenol derivatives, among them their simplified structures. This study examines the contribution of simplified structure for the antioxidant capacity of some natural and synthetic antioxidants. The resonance structures were related to the π-type electron system of carbon-carbon double bonds between both phenyl rings. Trans-resveratrol, phenyl-benzofuran, phenyl-indenone, and benzylidene-benzofuranone are the best basic antioxidant templates among the simplified derivatives studied here. Additionally, the stilbene moiety was found on the molecules with the best antioxidant capacity. Furthermore, our investigation suggests that these compounds can be used as antioxidant scaffold for designing and developing of new promising derivatives.
Methods
To investigate the structure–antioxidant capacity for sixteen simplified natural and proposed derivatives we have employed density functional theory and used Gaussian 09. Our DFT calculations were performed using the B3LYP functional and the 6-31+G(d,p) basis set. All electron transfer mechanisms were investigated by using values of HOMO, ionization potential, energy affinity, stabilization energies, and spin density distributions.





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Gaussian 09 software code G9S016832734579W-5269 N.
References
De S, Aamna B, Sahu R, Parida S, Behera SK, Dan AK (2022) Seeking heterocyclic scaffolds as antivirals against dengue virus. Eur J Med Chem 240:114576. https://doi.org/10.1016/j.ejmech.2022.114576
Haider K, Haider MR, Neha K, Yar MS (2020) Free radical scavengers: An overview on heterocyclic advances and medicinal prospects. Eur J Med Chem 204:112607. https://doi.org/10.1016/j.ejmech.2020.112607
Matos MJ, Vazquez-Rodriguez S, Fonseca A, Uriarte E, Santana L, Borges F (2017) Heterocyclic antioxidants in nature: coumarins. Curr Org Chem 21(4):311–324. https://doi.org/10.2174/1385272820666161017170652
Jeremić S, Amić A, Stanojević-Pirković M, Marković Z (2018) Selected anthraquinones as potential free radical scavengers and P-glycoprotein inhibitors. Org Biomol Chem 16(11):1890–1902. https://doi.org/10.1039/C8OB00060C
Weisburger JH (1999) Mechanisms of action of antioxidants as exemplified in vegetables, tomatoes and tea. Food Chem Toxicol 37(9-10):943–948. https://doi.org/10.1016/s0278-6915(99)00086-1
Sui G, Li T, Zhang B, Wang R, Hao H, Zhou W (2021) Recent advances on synthesis and biological activities of aurones. Bioorg Med Chem 1(29):115895. https://doi.org/10.1016/j.bmc.2020.115895
Panda SS, Chand M, Sakhuja R, Jain SC (2013) Xanthones as potential antioxidants. Curr Med Chem 20(36):4481–4507. https://doi.org/10.2174/09298673113209990144
Matos MJ, Mura F, Vazquez-Rodriguez S, Borges F, Santana L, Uriarte E, Olea-Azar C (2015) Study of coumarin-resveratrol hybrids as potent antioxidant compounds. Molecules 20(2):3290–3308. https://doi.org/10.3390/molecules20023290
Yamagata K, Yamori Y (2020) Inhibition of endothelial dysfunction by dietary flavonoids and preventive effects against cardiovascular disease. J Cardiovasc Pharmacol 75(1):1–9. https://doi.org/10.1097/FJC.0000000000000757
Fang X, Hu X (2018) Advances in the synthesis of lignan natural products. Molecules 23(12):3385. https://doi.org/10.3390/molecules23123385
Rajeshwari KC, Hiremathad A, Singh M, Santos MA, Keri RS (2017) A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives. Pharmacol Rep 69:281–295. https://doi.org/10.1016/j.pharep.2016.11.007
Thuy PT, Trang NV, Son NT (2020) Antioxidation of 2-phenylbenzofuran derivatives: structural-electronic effects and mechanisms. RSC Advances 10(11):6315–6332. https://doi.org/10.1039/C9RA10835A
Seery MK, Draper SM, Kelly JM, McCabe T, McMurry TBH (2005) The synthesis, structural characterization and photochemistry of some 3-phenylindenones. Synthesis 3:470–474. https://doi.org/10.1055/s-2005-861799
Miao Y, Hu Y, Yang J, Liu T, Sun J, Wang X (2019) Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Advances 9(47):27510–27540. https://doi.org/10.1039/C9RA04917G
Patel S, Rathod B, Regu S, Chak S, Shard A (2020) A perspective on synthesis and applications of fluorenones. Chem Select 5(34):10673–10691. https://doi.org/10.1002/slct.202002695
Hayyan M, Hashim MA, AlNashef IM (2016) Superoxide ion: generation and chemical implications. Chem Rev 116(5):3029–3085. https://doi.org/10.1021/acs.chemrev.5b00407
Gulcin İ (2020) Antioxidants and antioxidant methods: an updated overview. Arch Toxicol 94(3):651–715. https://doi.org/10.1007/s00204-020-02689-3
Harjumäki R, Pridgeon CS, Ingelman-Sundberg M (2021) CYP2E1 in alcoholic and non-alcoholic liver injury. Roles of ROS, reactive intermediates and lipid overload. Int J Mol Sci 22(15):8221–8240. https://doi.org/10.3390/ijms22158221
Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G, Mann GE, Pamplona R, Poli G, Portero-Otin M, Riahi Y, Salvayre R, Sasson S, Serrano J, Shamni O, Siems W, Siow RCM, Wiswedel I, Zarkovic K, Zarkovic N (2010) Pathological aspects of lipid peroxidation. Free Rad Res 44(10):1125–1171. https://doi.org/10.3109/10715762.2010.498478
Albuquerque RV, Malcher NS, Amado LL, Coleman MD, dos Santos DC, Borges RS, Valente SAS, Valente VC, Monteiro MC (2015) In vitro protective effect and antioxidant mechanism of resveratrol induced by dapsone hydroxylamine in human cells. PLoS ONE 10(8):e0134768. https://doi.org/10.1371/journal.pone.0134768
Galano A (2017) Free radicals induced oxidative stress at a molecular level. J Mex Chem Soc 59(4):231–262. https://www.scielo.org.mx/pdf/jmcs/v59n4/v59n4a2.pdf
Han KH, Hashimoto N, Fukushima M (2016) Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes. World J Gastroent 22(1):37–49. https://doi.org/10.3748/wjg.v22.i1.37
Kawamura T, Muraoka I (2018) Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants 7(9):119–137. https://doi.org/10.3390/antiox7090119
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53(6):1841–1856. https://doi.org/10.1021/jf030723c
Santos KLB, Bragança VAN, Pacheco LV, Ota SSB, Aguiar CPP, Borges RS (2022) Essential features for antioxidant capacity of ascorbic acid (vitamin C). J Mol Model 28(1):1–8. https://doi.org/10.1007/s00894-021-04994-9
Ou-Yang SS, Lu JY, Kong XQ, Liang ZJ, Luo C, Jiang H (2012) Computational drug discovery. Acta Pharmacol Sin 33(9):1131–1140. https://doi.org/10.1038/aps.2012.109
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacog Rev 4(8):118–126. https://doi.org/10.4103/0973-7847.70902
Palheta IC, Ferreira LR, Vale JKL, Silva OPP, Herculano AM, Oliveira KRHM, Chaves Neto AMJ, Campos JM, Santos CBR, Borges RS (2020) Alkylated sesamol derivatives as potent antioxidants. Molecules 25(14):3300. https://doi.org/10.3390/molecules25143300
das Neves PAPFG, Lobato CC, Ferreira LR, Bragança VAN, Veiga AAS, Ordoñez ME, Barros VA, de Aguiar CPO, Borges RS (2020) Molecular modification approach on kojic acid derivatives as antioxidants related to ascorbic acid. J Mol Model 26(11):318–326. https://doi.org/10.1007/s00894-020-04580-5
Tsolaki E, Nobelos P, Geronikaki A, Rekka EA (2014) Selected heterocyclic compounds as antioxidants. Synthesis and biological evaluation. Curr Top Med Chem 14(22):2462–2477. https://doi.org/10.2174/1568026614666141203120425
Adelusi TI, Akinbolaji GR, Yin X, Ayinde KS, Olaoba OT (2021) Neurotrophic, anti-neuroinflammatory, and redox balance mechanisms of chalcones. Eur J Pharmacol 15(891):173695. https://doi.org/10.1016/j.ejphar.2020.173695
Alara OR, Abdurahman NH, Ukaegbu CI (2021) Extraction of phenolic compounds: a review. Curr Res Food Sci 4:200–214. https://doi.org/10.1016/j.crfs.2021.03.011
Cramer J, Sager CP, Ernst B (2019) Hydroxyl groups in synthetic and natural-product-derived therapeutics: a perspective on a common functional group. J Med Chem 62(20):8915–8930. https://doi.org/10.1021/acs.jmedchem.9b00179
Schaub J, Zielesny A, Steinbeck C, Sorokina M (2020) Too sweet: cheminformatics for deglycosylation in natural products. J Cheminform 12:67. https://doi.org/10.1186/s13321-020-00467-y
Guzmán-Ávila R, Avelar M, Márquez EA, Rivera-Leyva JC, Mora JR, Flores-Morales V, Rivera-Islas J (2021) Synthesis, in vitro, and in silico analysis of the antioxidative activity of dapsone imine derivatives. Molecules 26(19):5747. https://doi.org/10.3390/molecules26195747
Charlton NC, Mastyugin M, Török B, Török M (2023) Structural features of small molecule antioxidants and strategic modifications to improve potential bioactivity. Molecules 28(3):1057. https://doi.org/10.3390/molecules28031057
Mendes APS, Borges RS, Chaves Neto AMJ, de Macedo LGM, da Silva ABF (2012) The basic antioxidant structure for flavonoid derivatives. J Mol Model 18(9):4073–4080. https://doi.org/10.1007/s00894-012-1397-0
Habgood M, James T, Heifetz A (2020) Conformational searching with quantum mechanic. Methods Mol Biol 2114:207–229. https://doi.org/10.1007/978-1-0716-0282-9_14
Maciel EN, Soares IN, da Silva SC, de Souza GLC (2019) A computational study on the reaction between fisetin and 2,2-diphenyl-1-picrylhydrazyl (DPPH). J Mol Model 25(4):103. https://doi.org/10.1007/s00894-019-3969-8
Nakajima M, Adachi Y, Nemoto T (2022) Publisher Correction: computation-guided asymmetric total syntheses of resveratrol dimers. Nat Communic 13(1):152–161. https://doi.org/10.1038/s41467-022-30160-7
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, revision C.01. Gaussian, Inc, Wallingford CT
Zhou B, Hu Z, Jiang Y, He X, Sun Z, Sun H (2018) Benchmark study of ionization potentials and electron affinities of armchair single-walled carbon nanotubes using density functional theory. J Phys Cond Mat 30(21):215501–215509. https://doi.org/10.1088/1361-648X/aabd18
Prasad VK, Otero-de-la-Roza A, DiLabio GA (2022) Small-basis set Density-Functional Theory methods corrected with atom-centered potentials. J Chem Theory Comput 18(5):2913–2930. https://doi.org/10.1021/acs.jctc.2c00036
Borges RS, Batista Jr J, Viana RB, Baetas AC, Orestes E, Andrade MA, Honório KM, da Silva ABF (2013) Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 18(10):12663–12674. https://doi.org/10.3390/molecules181012663
Tirado-Rives J, Jorgensen WL (2008) Performance of B3LYP Density Functional methods for a large set of organic molecules. J Chem Theory Comput 4(2):297–306. https://doi.org/10.1021/ct700248k
Yang Y, Ga H (2012) Comparison of DFT methods for molecular structure and vibration spectra of ofloxacin calculations spectrochim. Acta A Mol Biomol Spectrosc. 85(1):303–309. https://doi.org/10.1016/j.saa.2011.10.019
Zheng YZ, Deng G, Liang Q, Chen DF, Guo R, Lai RC (2017) Antioxidant activity of quercetin and its glucosides from propolis: a theoretical study. Sci Rep 7(1):7543. https://doi.org/10.1038/s41598-017-08024-8
Farrokhnia M (2020) Density Functional Theory studies on the antioxidant mechanism and electronic properties of some bioactive marine meroterpenoids: Sargahydroquionic acid and sargachromanol. ACS Omega 5(32):20382–20390. https://doi.org/10.1021/acsomega.0c02354
Leopoldini M, Rondinelli F, Russo N, Toscano M (2010) Pyranoanthocyanins: a theoretical investigation on their antioxidant activity. J Agric Food Chem 58(15):8862–8871. https://doi.org/10.1021/jf101693k
Yi Y, Adrjan B, Li J, Hu B, Roszak S (2019) NMR studies of daidzein and puerarin: active anti-oxidants in traditional Chinese medicine. J Mol Model 25(7):202. https://doi.org/10.1007/s00894-019-4090-8
Queiroz AN, Martins CC, Santos KLB, Carvalho ES, Owiti AO, Oliveira KRM, Herculano AM, Da Silva ABF, Borges RS (2020) Experimental and theoretical study on structure-tautomerism among edaravone, isoxazolone, and their heterocycles derivatives as antioxidants. Saudi Pharm J 28(7):819–827. https://doi.org/10.1016/j.jsps.2020.06.001
Vidhya V, Austine A, Arivazhagan M (2020) Molecular structure, aromaticity, vibrational investigation and dual descriptor for chemical reactivity on 1- chloroisoquinoline using quantum chemical studies. Results in Materials 6:100097. https://doi.org/10.1016/j.rinma.2020.100097
Bentes AL, Borges RS, Monteiro WR, de Macedo LGM, Alves CN (2016) Structure of dihydrochalcones and related derivatives and their scavenging and antioxidant activity against oxygen and nitrogen radical species. Molecules 16(2):1749–1760. https://doi.org/10.3390/molecules16021749
Queiroz AN, Gomes BAQ, Moraes Jr WM, Borges RS (2009) A theoretical antioxidant pharmacophore for resveratrol. Eur J Med Chem 44(4):1644–1649. https://doi.org/10.1016/j.ejmech.2008.09.023
Funding
The authors are grateful to CNPq for financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization, Rosivaldo S. Borges; data extraction, Christiane P. O. Aguiar and Nicole L. L. Oliveira; structure–reactivity, Joyce K. L. Vale; calculations, Israel N. A. Amaral; electronic properties, Antonio M. J. Chaves Neto; editing, Albérico B. F. da Silva; final version, Rosivaldo S. Borges.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Yes.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borges, R.S., Aguiar, C.P.O., Oliveira, N.L.L. et al. Antioxidant capacity of simplified oxygen heterocycles and proposed derivatives by theoretical calculations. J Mol Model 29, 232 (2023). https://doi.org/10.1007/s00894-023-05602-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05602-8