Abstract
Context
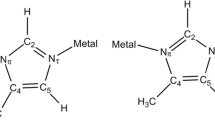
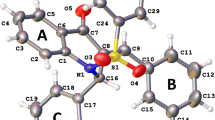
At present, sulfonamides and their metal complexes have received a new impetus for development. Of particular interest is the study of molecular and crystal structures, which takes into account weak non-valent interactions. Despite the low energy of such interactions, in many cases, they act collectively, and the sum of their actions can play a significant role. As a result, the spectrum of medical and biological activity of new metal complexes is expanded. In this regard, the synthesis and study of the molecular and crystal structure of sulfonamides and their metal complexes is of undoubted relevance. In this work, we studied non-valent intra- and intermolecular interactions in ligands of sulfonamide-substituted imidazo[2,1-b]thiazoles and their previously unknown complexes with CuCl2. The performed analysis of the data obtained by X-ray diffraction analysis made it possible to establish the intramolecular π-stacking interaction in imidazothiazole ligands, which is retained in their complexes with CuCl2. Within the framework of QTAIM topological analysis of electron density and DORI analysis, stereoelectronic and topological structures were studied. In the complexes, tetral, chalcogen, and pnycogen new interligand non-valent interactions were established. The energies of all established types of non-valent interactions have been calculated, and their comparative evaluation has been made.
Methods
X-ray data of new arylsulfonylamino-substituted derivatives of imidazo[2,1-b]thiazoles and their metal complexes with CuCl2 have been studied. To determine the theoretical prerequisites for the occurrence of π-stacking in the molecules under study, the QTAIM method was used in the framework of the DFT/B3LYP/6–311 + G(d) calculation using the GAUSSIAN 09 program. In addition, the DORI electron density region overlap indicator and the Multiwfn program were used to analyze non-valent interactions.














Similar content being viewed by others
Data availability
Not applicable.
References
Mironova EV, Lodochnikova OA, Krivolapov DB, Veremeichik Ya. V, Plemenkov VV, Litvinov IA (2014) J Struct Chem 55:539–547
Abbate F, Supuran CT, Scozzafava A, Orioli P, Stubbs MT, Klebe G (2002) J Med Chem 45:3583–3587
Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT (2003) Med Chem 10:925–953
Supuran CT, Scozzafava A (2000) Exp Opin Ther Pat 10:575–600
Huang D, Caflisch A (2012) J Chem Theory Comput 8(5):1786–1794
Liu J, Li A-R, Wang Y et al (2011) ACS Med Chem Lett 2(5):326
Miller MW, Basra S, Kulp DW et al (2009) PNAS 106(3):719
McGuire RT, Simon CM, Yadav AA, Ferguson MJ, Stradiotto M (2020) Angew Chem Int Ed Engl 59:8952–8956
Ovung A, Bhattacharyya J (2021) Biophys Rev 13:259–272
Mukherjee P, Woroch CP, Cleary L, Rusznak M, Franzese RW, Reese MR, Tucker JW, Humphrey JM, Etuk SM, Kwan SC, am Ende CW, Ball ND (2018) Org Lett 20:3943–3947
Vigorito A, Calabrese C, Maris A, Loru D, Peña I, Sanz EM, Melandri S (2022) Moleculs 27(9):2820
Andreani A, Burnelli S, Granaiola M, Guardigli M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Rizzoli M, Varoli L, Roda A (2008) Eur J Med Chem 43(3):657–661
Borhani DW, Calderwood DJ, Frank KE, Davis HM, Josephsohn NS, Skinner BS (2008) US Patent WO 063287
Emmitte KA, Wilson BJ, Baum EW, Emerson HK, Kuntz KW, Nailor KE, Salovich JM, Smith SC, Cheung M, Gerding RM, Stevens KL, Uehling DE, Mook RA Jr, Moorthy GS, Dickerson SH, Hassell AM, Leesnitzer MA, Shewchuk LM, Groy A, Rowand JL, Anderson K, Atkins CL, Yang J, Sabbatini P, Kumar R (2009) Bioorg Med Chem Lett 19(3):1004–1007
Fidanze SD, Erickson SA, Wang GT, Mantei R, Clark RF, Sorensen BK, Bamaung NY, Kovar P, Johnson EF, Swinger KK, Stewart KD, Zhang Q, Tucker LA, Pappano WN, Wilsbacher JL, Wang J, Sheppard GS, Bell RL, Davidsen SK, Hubbard RD (2010) Bioorg Med Chem Lett 20(8):2452–2455
Andreani A, Rambaldi M, Mascellani G, Rugarli P (1987) Eur J Med Chem 22(1):19–22
Andreani A, Rambaldi M, Andreani F, Bossa R, Galatulas I (1988) Eur J Med Chem 23(4):385–389
Andreani A, Rambaldi M, Locatelli A, Bossa R, Fraccari A, Galatulas I (1993) Pharm. Acta Helv 68(1):21–24
Andreani A, Bonazzi D, Rambaldi M (1982) Arch Pharm 315(5):451–456
Andreani A, Rambaldi M, Locatelli A, Bossa R, Fraccari A, Galatulas I (1992) J Med Chem 35(24):4634–4637
Poorrajab F, Ardestani SK, Emami S, Behrouzi-Fardmoghaddam M, Shafiee A, Foroumadi A (2009) Eur J Med Chem 44(4):1758–1762
Khalaj A, Nakhjiri M, Negahbani AS, Samadizadeh M, Firoozpour L, Rajabalian S, Samadi N, Faramarzi MA, Adipour N, Shafiee A, Foroumadi A (2011) Eur J Med Chem 46(1):65–70
Gupta GD, Jain KK, Gupta RP, Pujari HK (1983) Indian J Chem Sect B: Org Chem Incl Med Chem 22:268
Amarouch H, Loiseau PR, Bacha C, Caujolle R, Payard M, Loiseau PM, Bories C, Gayral P (1987) Eur J Med Chem 22(5):463–466
Muhammad P, Aqsa R, Anfal M, Zohaib S, Shah H, Ayoub R, Umar Y, Ahmad A (2020) J Mol Struct 1202:127284
Sekhar EV, Karki SS, Rangaswamy J, Bhat M, Sujeet K (2021) Beni-Suef Univ J Basic Appl Sci 10(1):28
Al-khodir (2015) Orient J Chem 31(3):1277–1285
Huentupil Y, Pena L, Novoa N, Berrino E, Arancibia R, Supuran CT (2019) J Enzym Inhibit Med Chem 34(1):451–458
Serkan D, Nilgun OK, Daran J-C, Labande A, Polib R (2013) Eur J Inorg Chem 2013:3224–3232
Szadkowska A, Zukowska K, Pazio AE, Wozniak K, Kadyrov R, Grela K (2011) Organometallics 30:1130–1138
Jin W, Li X, Wan B (2011) J Org Chem 76:484–491
Javier C, Gloria A, Sacramento F, Julio L, Jose A (2000) Ramırez; Joaquın, Borras. Inorg Chim Acta 304:170–177
Yan M, Li T, Yang Z (2011) Inorg Chem Commun 14:463–465
Burlov AS, Vlasenko VG, Koshchienko Yu.V, Nikolaevskii SA, Kiskin MA, Minin VV, Ugolkova EA, Efimov NN, Bogomyakov AS, Kolodina AA, Zubavichus Ya.V, Levchenkov SI, Garnovskii DA (2018) Polyhedron 154:123–131
Sajjad, Hussain, Sumrra; Abrar, Ul, Hassan; Muhammad, Nadeem, Zafar; Syed, Salman, Shafqat; Ghulam, Mustafa; Muhammad, Naveed, Zafar; Muhammad, Zubair; Muhammad, Imran (2022) J Mol Struct 1250:131710
Serykh VYu, Kaliev AR, Ushakov IA, Borodina TN, Smirnov VI, Rozentsveig IB (2018) Arkivoc Part III:62–75
Borodina TN, Smirnov VI, Serykh VY, Rozentsveig IB (2022) J Mol Struct 1248:131423
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann HJ (2009) Appl Cryst 42:339–341
Sheldrick GM (2008) Acta Crystallogr Sect A Found Adv A64:112–122
Bruker (2001) SADABS. Bruker AXS Inc., Madison, Wisconsin, USA., (n.d.)
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc. Wallingford CT
Bader RFW, Matta CF (2013) Found Chem 15(3):253–276
Keith TA (2011) AIMALL. TK Gristmill Software. Overland Park KS. USA
Lu T, Chen FWJ (2012) of Computat. Chem 33(5):580–592
Parkin A, Collins A, Gilmore CJ, Wilson CC (2008) Acta Crystallogr B 64:66–71
Rozentsveig IB, Serykh VY, Chernysheva GN, Chernyshev KA, Kondrashov EV, Tretyakov EV, Romanenko GV (2013) Eur J Org Chem 368–375
Zahid HC (2008) J Enzyme Inhibition Med Chem 23(1):120–130
Goszczycki P, Stadnicka K, Brela MZ, Grolik J, Ostrowska K (2017) J Mol Struct 1146:337–346
De Silva P, Corminboeuf C (2014) J Chem Theor Computat 10(9):3745–3756
Espinosa E, Molins E, Lecomte C (1998) Chem Phys Lett 285(3–4):170–173
Acknowledgements
The work was carried out using the material and technical base of the Baikal Analytical Center for Collective Use of the SB RAS. This work was carried out within the framework of the research project of Russian Academy of Sciences no. 121021000264-1.
Author information
Authors and Affiliations
Contributions
Borodina T.N.: data curation, writing — original draft. Smirnov V.I.: conceptualization, methodology, writing — reviewing and editing. Serykh V.Yu.: resources. Rozentsveig I.B.: writing — reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borodina, T.N., Smirnov, V.I., Serykh, V.Y. et al. Structural and theoretical study of π-stacking interactions in new complexes based on CuCl2 and 3-sulfonamide-substituted imidazo[2,1-b]thiazoles. J Mol Model 29, 136 (2023). https://doi.org/10.1007/s00894-023-05549-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05549-w