Abstract
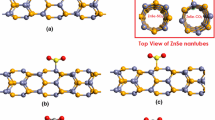
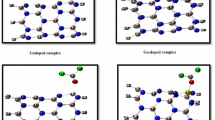
Adsorption of pollutant gas molecules (NO2, SO2, and O3) on the surface of the Al-doped stanene nanotube was investigated within the first principle calculations of density functional theory (DFT). Adsorption mechanisms were studied by analyzing optimized structures, band structures, projected density of states (PDOS), charge density difference (CDD), molecular orbitals, and band theory. Investigation of charge transfer by Mulliken population showed that NO2 accumulated while SO2 and O3 depleted charge density on the Al-doped nanotube. The differences in band structures before and after adsorption implied that the electronic characteristics of Al-doped nanotube changed dramatically in case of NO2 adsorption, which converted Al-doped nanotube to a semiconductor material. High adsorption energy and the significant overlap between PDOS spectra indicated that the adsorption process was chemisorption for NO2, SO2, and O3 on the doped nanotube with the obtained order of O3 > SO2 > NO2. The results showed that the adsorption of NO2, SO2, and O3 occurred on the Al-doped stanene nanotube, and that all the three gas molecules could be detected by Al-doped stanene nanotube with various detection strengths.













Similar content being viewed by others
References
Brauer M, Casadei B, Harrington RA, Kovacs R, Sliwa K (2021) Taking a stand against air pollution—the impact on cardiovascular disease. J Am Coll Cardiol 77(13):1684–1688
Pandey A, Brauer M, Cropper M, Balakrishnan K, Mathur P, Dey S, Turkgulu B, Kumar GA, Khare M, Beig G (2021) Health and economic impact of air pollution in the states of India: the Global Burden of Disease Study 2019. Lancet Planet Health 5:e25–e38
Münzel T, Hahad O, Daiber A (2021) Running in polluted air is a two-edged sword —physical exercise in low air pollution areas is cardioprotective but detrimental for the heart in high air pollution areas. Eur Heart J 42:2498–2500
Chi C, Cristaldi A, Fiore M, Grasso A, Zuccarello P, Signorelli SS, Conti GO, Ferrante M (2020) The role of air pollution (PM and NO 2) in COVID-19 spread and lethality: a systematic review. Environ Res 191:1102109
Yogi R, Jaiswal NK (2019) Adsorption of CO gas molecules on zigzag BN/AlN nanoribbons for nano sensor applications. Phys Lett 383:532–538
Gillespie-Bennett J, Pierse N, Wickens K, Crane J, Howden-Chapman P, the Housing Heating and Health Study Research Team (2011) The respiratory health effects of nitrogen dioxide in children with asthma. Eur Respir J 38:303–309
Faustini A, Rapp R, Forastiere F (2014) Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur Respir J 44:744–53
Pattenden S, Hoek G, Braun-Fahrländer C, Forastiere F, Kosheleva A, Neuberger M, Fletcher T (2006) NO2 and children’s respiratory symptoms in the PATY study. Occup Environ Med 63:828–835
He W, Zhao Y, Xiong Y (2020) Bilayer polyaniline−wo3thin-film sensors sensitive to NO2. ACS Omega 5:9744–9751
Levy JI, Lee K, Yanagisawa Y, Eng D, Hutchinson P, Spengler JD (1999) Determinants of nitrogen dioxide concentrations in indoor ice skating rinks. Am J Public Health 88:1781–1786
Erglund B, Etal M (1993) Health risk evaluation of nitrogen oxides. Scand J Work Environ Health 75:14
Lin W, Brunekreef B, Gehring U (2013) Meta-analysis of the effects of indoor nitrogen dioxide and gas cooking on asthma and wheeze in children. Int J Epidemiol 42:1724–1737
Ye Z, Duan Ch, Sheng R, Xu J, Wang H, Zeng L (2018) A novel colorimetric and ratiometric fluorescent probe for visualizing SO2 derivatives in environment and living cells. Talanta 176:389–396
Martínez-Ahumada E, Pez-Olvera AL, Jancik V, Sánchez-Bautista JE, Lez-Zamora EG, Martis V, Williams D, Ibarra IA (2020) MOF materials for the capture of highly toxic H2S and SO2. Organometallics 39:883–915
Van Tong Ph, Hoa ND, Th NH, Van Duy N, Hung ChM, Van Hieu N (2018) SO2 and H2S sensing properties of hydrothermally synthesized CuO nanoplates. J Electron Mater 47:7170–7178
Buadong D, Jinsart W, Funatagawa I, Karita K, Yano E (2009) Association between PM 10 and O3 levels and hospital visits for cardiovascular diseases in Bangkok, Thailand. J Epidemiology 19:182–188
Xue Ch, Ye C, Zhang Ch, Catoire V, Liu P, Gu R, Zhang J, Ma Zh, Xi Z, Zhang W, le Krysztofiak YRG, Tong Sh, Xue L, An J, Ge M, Mellouki A, Mu Y (2021) Evidence for strong HONO emission from fertilized agricultural fields and its remarkable impact on regional O3 pollution in the Summer North China Plain. ACS Earth Space Chem 5:340–347
Valerio F, Stella A, Munizzi A (2000) Correlations between PAHs and CO, NO, NO2, O3 along an urban street. Taylor & Francis 20:235–244
Triantafyllou AG, Zoras S, Evagelopoulos V, Garas S (2008) PM10, O3, CO concentrations and elemental analysis of airborne particles in a school building. Water Air Soil Pollut 8:77–87
Pathak RK, Presto AA, Lane TE, Stanier CO, Donahue NM, Pandis SN (2007) Ozonolysis of α-pinene: parameterization of secondary organic aerosol mass fraction. Atmos Chem Phys 7:3811–3821
Avise J, Chen J, Lamb B, Wiedinmyer C, Guenther A, Salathé E, Mass C (2009) Attribution of projected changes in summertime US ozone and PM 2.5 concentrations to global changes. Atmos Chem Phys 9:1111–1124
Calfapietra C, Fares S, Loreto F (2009) Volatile organic compounds from Italian vegetation and their interaction with ozone. Environ Pollut 157:1478–1486
Calfapietra C, Manes FS, Morani A, Sgrigna G, Loreto F (2013) Role of Biogenic Volatile Organic Compounds (BVOC) emitted by urban trees on ozone concentration in cities: a review. Environ Pollut 183:71–80
Streng AG (1961) Tables of Ozone Properties. J Chem Eng Data J CHEM ENG DATA 6:431–436
Guo JJ, Fiore AM, Murray LT, Jaffe DA, Schnell JL, Moore ChT, Milly GP (2018) Average versus high surface ozone levels over the continental USA: model bias, background influences, and interannual variability. Atmos Chem Phys 18:12123–12140
Bell ML, Peng RD, Dominici F (2006) The exposure–response curve for ozone and risk of mortality and the adequacy of current ozone regulations. Environ Health Perspect 114:532–536
Nagarajan V, Chandiramouli R (2018) A novel approach for detection of NO2 and SO2 gas molecules using graphane nanosheet and nanotubes - a density functional application. Diam Relat Mater 85:0925–9635
Salih E, A. Ayesh I, (2018) CO, CO2, and SO2 detection based on functionalized graphene nanoribbons: First principles study. Diam Relat Mater 85:53–62
Zhao Y, Hong H, Gong Q, Ji L (2013) 1D Nanomaterials: synthesis, properties, and applications. J Nanomater 2013:1687–4110
Machín A, Fontánez K, Arango JC, Ortiz D, De León J, Pinilla S, Nicolosi V, Petrescu FI, Morant C, Márquez F (2021) One-Dimensional (1D) Nanostructured materials for energy applications. Materials 14:2609
Ansón-Casaos A, Ciria JC, Sanahuja-Parejo O, Vı́ctor-Román S, González-Domı́nguez JM, Garcı́a-Bordejé E, Benito AM, Maser WK (2020) The viscosity of dilute carbon nanotube (1D) and graphene oxide (2D) nanofluids. Phys Chem Chem Phys 22:11474
De Sousa JM, Bizao RA, Sousa Filho VP, Aguiar AL, Coluci VR, Pugno NM, Girao EC, Souza Filho AG, Galvao DS (2019) Elastic properties of graphyne-based nanotubes. Mater Sci 1. https://doi.org/10.48550/arXiv.1905.02104
Srinivasu K, Ghosh SK (2012) Graphyne and graphdiyne: promising materials for nanoelectronics and energy storage applications. J Phys Chem C 116:5951–5956
Abbasi A, Jahanbin Sardroodi J (2018) Interaction of sulfur trioxide molecules with armchair and zigzag stanene-based nanotubes: electronic properties exploration by DFT calculations. Adsorption 24:443–458
Long RQ, Yang RT (2001) Carbon nanotubes as a superior sorbent for nitrogen oxides. Ind Eng Chem Res 40:4288–4291
Ellison MD, Crotty MJ, Koh D, Spray RL, Tate KE (2004) Adsorption of NH3 and NO2 on single-walled carbon nanotubes. J Phys ChemB 108:7938–7943
Zhang X, Yang B, Wang X, Luo Ch (2012) Effect of plasma treatment on multi-walled carbon nanotubes for the detection of H2S and SO2. Sensors 12:9375–9385
Mittal M, Kumar A (2014) Carbon nanotube (CNT) gas sensors for emissions from fossil fuel burning. Sens Actuators B Chem 203:349–362
Hoang ND, Van Cat V, Nam MH, Phan VN, Le AT, Van Quy N (2019) Enhanced SO2 sensing characteristics of multi-wall carbon nanotubes based mass-type sensor using two-step purification process. Sens Actuators A 295:696–702
Abbasi A, Jahanbin Sardroodi J, Rastkar Ebrahimzadeh A, Yaghoobi M (2018) Theoretical study of the structural and electronic properties of novel stanene-based buckled nanotubes and their adsorption behaviors. Appl Surf Sci 435:733–742
Abbasi A, Jahanbin Sardroodi J (2018) Structural and electronic properties of group-IV tin nanotubes and their effects on the adsorption of SO2 molecules: insights from DFT computations. Int J Appl Phys 124:165302
Sonawane MR, Nagare BJ, Habale D, Shivade RK (2013) Comparative study of adsorption of O2, CO2, NO2 and SO2 on pristine and Si-Doped Carbon Nanotubes. Open J Adv Mater Res 678:179–184
Atram RG, Sonawane MR (2019) Comparative study of adsorption of ozone molecule on pristine and Si doped single wall carbone nanotube by density functional theory. Mater Phys Mech 42:1605–8119
Zhang H-P, Hou J-L, Wang Y, Tang P-P, ZhangY-P L-Y, Liu Ch, Tang Y (2017) Adsorption behavior of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin on pristine and doped black phosphorene: a DFT study. Chemosphere 185:509–517
Manzhos S (2020) Machine learning for the solution of the Schrödinger equation. Mach Learn: Sci Technol 1:013002
Koch O, Kreuzer W, Scrinzi A (2006) Approximation of the time-dependent electronic Schrödinger equation by MCTDHF. Appl Math Comput 173:960–976
Romera E, Dehesa JS (1994) Weizsäcker energy of many-electron systems. Phys Rev A 50:256
Artacho E, Gale JD, García A, Junquera V, Ordejón P, Sánchez-Portal D (2002) The SIESTA method for ab initio order-N materials simulation. J Condens Matter Phys 14:0953–8984
Shao X, Mi W, Pavanello M (2021) GGA-level subsystem DFT achieves Sub-kcal/mol accuracy intermolecular interactions by mimicking nonlocal functionals. J Chem Theory Comput 7:3455–3461
González Ramirez IA, Alcalá Varilla LA, Montoya JA (2019) A DFT study about the effects of exchange-correlation functional on the structural and electronic properties of Anatase. J Phys: Conf Ser 1219:7–9
Hammer B, Hansen LB, Nørskov JK (1999) Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys Rev B 59:7413
Skriganov MM (1987) Brillouin zones and the geometry of numbers. J Sov Math 36:140–154
Dion M, Jacobson N, Schröder E, Hyldgaard P, Simak S, Langreth DC, Lundqvist BI (2003) Van der Waals density functional for layered structures. Phys Review lett 91:126402
Dion M, Rydberg H, Schröder E, Langreth DC, Lundqvist BI (2004) Van der Waals density functional for general geometries. Phys Review lett 92:246401
Amft M, Lebègue S, Eriksson O, Skorodumova NV (2011) Adsorption of Cu, Ag, and Au atoms on graphene including van der Waals interactions. J Condens Matter Phys 23:395001
Motaee A, Javadian S (2021) Khosravian M, Influence of adsorption energy in graphene production via surfactant-assisted exfoliation of graphite: a graphene-dispersant design. ACS Appl Nano Mater 4:3545–3556
Wang B, Wang ShL, Truhlar DG (2014) Modeling the partial atomic charges in inorganometallic molecules and solids and charge redistribution in lithium-ion cathodes. J Chem Theory Comput 10:5640–5650
Bo Z, Guo X, Wei X, Yang H, Yan J, Cen K (2019) Density functional theory calculations of NO2 and H2S adsorption on the group 10 transition metal (Ni, Pd and Pt) decorated graphene. Phys E Low-dimens Syst Nanostruct 109:156–163
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions. J Chem Phys 23:1833–1840
Xi Xu, Li J, Zhang Xu, Xu H, Ke ZhF, Zhao C (2015) Removal of NO with silicene: a DFT investigation. RSC Adv 5:22135
Liu H, Xu L, Gui Y, Ran L, Chen X (2021) Adsorption properties of Ag 2 O-MoSe 2 towards SF 6 decomposed products. Vacuum 189:110248
Herzberg G (1966) Electronic spectra and electronic structure of polyatomic molecules. Van Nostrand, New York
Pan HR, Chen HJ, Wu ZH, Ge P, Ye Sh, Lee GH, Hsu HF (2021) Structural and spectroscopic evidence for a side-on Fe(III)–superoxo complex featuring discrete O-O bond distances. JACS Au 1:1389–1398
Komorowski L (1987) Chemical Hardness and L. Pauling’s scale of electronegativity. Zeitschrift für Naturforschung A 42:767–773
Tozini D, Forti M, Gargano P, Alonso PR, Rubiolo GH (2015) Charge difference calculation in Fe/Fe3 O4 interfaces from DFT results. Procedia Mater Sci 9:612–618
Prats H, Stamatakis M (2022) Atomistic and electronic structure of metal clusters supported on transition metal carbides: implications for catalysis. J Mater Chem A 10:1522–1534
Author information
Authors and Affiliations
Contributions
Nafiseh Karimi wrote the main text manuscript, performed all DFT calculations, and reviewed the manuscript. Jaber Jahanbin Sardroodi: supervision 1. Alireza Ebrahimzadeh Rastkar: supervision 2. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karimi, N., Sardroodi, J.J. & Rastkar, A.E. The adsorption of NO2, SO2, and O3 molecules on the Al-doped stanene nanotube: a DFT study. J Mol Model 28, 290 (2022). https://doi.org/10.1007/s00894-022-05296-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05296-4