Abstract
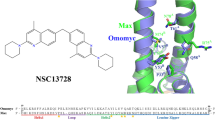
Myc is a bHLHZip protein involved in growth control and cancer, which does not form a homodimer. Myc operates in a network with its heterodimerization partner Max, the latter of which can form homodimer and heterodimer. Omomyc, a polypeptide, can block Myc to treat cancers because it can both homodimerize as efficiently as Max and heterodimerize with both Myc and Max. However, the binding efficiencies to DNA for the mentioned two homodimers (Omomyc-Omomyc and Max-Max) and three heterodimers (Myc-Max, Omomyc-Myc, and Omomyc-Max) are still controversial. By molecular dynamics simulations and MM/GBSA free energy calculation, we ranked the binding affinities of five dimers to DNA and analyzed the contribution of single amino acids to the molecular recognition of dimers to DNA. Our simulation showed that the Omomyc-Omomyc dimer exhibited the highest binding energy to DNA, followed by the Omomyc-Myc, Max-Max, Omomyc-Max, and Myc-Max dimers. Moreover, five Arg residues (i.e., 7, 8, 15, 17, and 18 numbered by Omomyc) and five Lys residues (i.e., 6, 22, 40, 43, and 48 numbered by Omomyc) dominated the binding of various dimers to DNA while the residues Asp23 and Asp37 weakened the affinities via repulsive interaction. Our simulation would provide worthy information for further development of the structure-based design of novel Omomyc-like peptide inhibitors against Myc in the future.
Graphical abstract









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Carroll PA, Freie BW, Mathsyaraja H, Eisenman RN (2018) The MYC transcription factor network: balancing metabolism, proliferation and oncogenesis. Front Med 12(4):412–425. https://doi.org/10.1007/s11684-018-0650-z
Dejure FR, Eilers M (2017) MYC and tumor metabolism: chicken and egg. EMBO J 36(23):3409–3420. https://doi.org/10.15252/embj.201796438
Perez-Olivares M, Trento A, Rodriguez-Acebes S, Gonzalez-Acosta D, Fernandez-Antoran D, Roman-Garcia S, Martinez D, Lopez-Briones T, Torroja C, Carrasco YR, Mendez J, Moreno de Alboran I (2018) Functional interplay between c-Myc and Max in B lymphocyte differentiation. EMBO Rep 19(10):e45770. https://doi.org/10.15252/embr.201845770
Luo W, Chen J, Li L, Ren X, Cheng T, Lu S, Lawal RA, Nie Q, Zhang X, Hanotte O (2019) c-Myc inhibits myoblast differentiation and promotes myoblast proliferation and muscle fibre hypertrophy by regulating the expression of its target genes, miRNAs and lincRNAs. Cell Death Differ 26(3):426–442. https://doi.org/10.1038/s41418-018-0129-0
Melnik S, Werth N, Boeuf S, Hahn EM, Gotterbarm T, Anton M, Richter W (2019) Impact of c-MYC expression on proliferation, differentiation, and risk of neoplastic transformation of human mesenchymal stromal cells. Stem Cell Res Ther 10(1):73. https://doi.org/10.1186/s13287-019-1187-z
Boone DN, Hann SR (2011) The Myc-ARF-Egr1 pathway: unleashing the apoptotic power of c-Myc. Cell Cycle 10(13):2043–2044. https://doi.org/10.4161/cc.10.13.15711
Wang XN, Su XX, Cheng SQ, Sun ZY, Huang ZS, Ou TM (2019) MYC modulators in cancer: a patent review. Expert Opin Ther Pat 29(5):353–367. https://doi.org/10.1080/13543776.2019.1612878
Park S, Chung S, Kim KM, Jung KC, Park C, Hahm ER, Yang CH (2004) Determination of binding constant of transcription factor myc-max/max-max and E-box DNA: the effect of inhibitors on the binding. Biochim Biophys Acta 1670(3):217–228. https://doi.org/10.1016/j.bbagen.2003.12.007
Blackwood EM, Eisenman RN (1991) Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 251(4998):1211–1217. https://doi.org/10.1126/science.2006410
Soucek L, Helmer-Citterich M, Sacco A, Jucker R, Cesareni G, Nasi S (1998) Design and properties of a Myc derivative that efficiently homodimerizes. Oncogene 17(19):10. https://doi.org/10.1038/sj.onc.1202199
Masso-Valles D, Soucek L (2020) Blocking Myc to treat cancer: reflecting on two decades of Omomyc. Cells 9(4):883. https://doi.org/10.3390/cells9040883
Jung LA, Gebhardt A, Koelmel W, Ade CP, Walz S, Kuper J, von Eyss B, Letschert S, Redel C, d’Artista L, Biankin A, Zender L, Sauer M, Wolf E, Evan G, Kisker C, Eilers M (2017) OmoMYC blunts promoter invasion by oncogenic MYC to inhibit gene expression characteristic of MYC-dependent tumors. Oncogene 36(14):1911–1924. https://doi.org/10.1038/onc.2016.354
Nair SK, Burley SK (2003) X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors. Cell 112(2):193–205. https://doi.org/10.1016/s0092-8674(02)01284-9
Ferré-D’Amaré AR, Prendergast GC, Ziff EB, Burley SK (1993) Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature 363(6424):38–45. https://doi.org/10.1038/363038a0
Case DA, Cheatham TE 3rd, Darden T, Gohlke H, Luo R, Merz KM Jr, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26(16):1668–1688. https://doi.org/10.1002/jcc.20290
Duan Y, Wu C, Chowdhury S, Lee MC, Xiong GM, Zhang W, Yang R, Cieplak P, Luo R, Lee T, Caldwell J, Wang JM, Kollman P (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem 24(16):1999–2012. https://doi.org/10.1002/jcc.10349
Gao J, Liang L, Chen Q, Zhang L, Huang T (2018) Insight into the molecular mechanism of yeast acetyl-coenzyme A carboxylase mutants F510I, N485G, I69E, E477R, and K73R resistant to soraphen A. J Comput Aided Mol Des 32(4):547–557. https://doi.org/10.1007/s10822-018-0108-z
Chen H, Wang Y, Gao Z, Yang W, Gao J (2019) Assessing the performance of three resveratrol in binding with SIRT1 by molecular dynamics simulation and MM/GBSA methods: the weakest binding of resveratrol 3 to SIRT1 triggers a possibility of dissociation from its binding site. J Comput-Aided Mol Des 33(4):437–446. https://doi.org/10.1007/s10822-019-00193-0
Shi S, Wang Q, Liu S, Qu Z, Li K, Geng X, Wang T, Gao J (2021) Characterization the performances of twofold resveratrol integrated compounds in binding with SIRT1 by molecular dynamics simulation and molecular mechanics/generalized born surface area (MM/GBSA) calculation. Chem Phys 544:111108. https://doi.org/10.1016/j.chemphys.2021.111108
Onufriev A, Bashford D, Case DA (2000) Modification of the generalized born model suitable for macromolecules. J Phys Chem B 104(15):3712–3720. https://doi.org/10.1021/jp994072s
Weiser J, Shenkin PS, Still WC (1999) Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J Comput Chem 20(2):217–230. https://doi.org/10.1002/(SICI)1096-987X(19990130)20:2%3c217::AID-JCC4%3e3.0.CO;2-A
Gao J, Cui W, Du Y, Ji M (2013) Insight into the molecular mechanism about lowered dihydrofolate binding affinity to dihydrofolate reductase-like 1 (DHFRL1). J Mol Model 19(12):5187–5198. https://doi.org/10.1007/s00894-013-2018-2
Funding
This work was supported by the Six Talent Peaks Project in Jiangsu Province (grant number YY-046), the Qinglan Project of Jiangsu Province of China, and Jiangsu Training Programs of Innovation and Entrepreneurship for Undergraduates (grant number 202110313022Z).
Author information
Authors and Affiliations
Contributions
Jian Gao and Yuxin Dai performed the molecular dynamics simulations; Jinyuan Zhang, Yinchuan Wang, and Yuxin Dai performed the MM/GBSA binding free energy calculations; Jian Gao and Linlin Liu analyzed the data and wrote the paper.
Corresponding authors
Ethics declarations
Ethics approval
We did not perform any experiments when preparing this article, so neither ethics review nor informed consent was necessary.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, Y., Zhang, J., Wang, Y. et al. Computational insights into the differentiated binding affinities of Myc, Max, and Omomyc dimers to the E-boxes of DNA. J Mol Model 28, 329 (2022). https://doi.org/10.1007/s00894-022-05261-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05261-1