Abstract
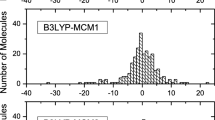
The domain of application of the G3(MP2)//B3-SBK theory was expanded, and its efficiency was evaluated to determinate enthalpies of formation of forty-one iodine compounds. The results were compared to those obtained with the G2 theory for the same set of molecules. The G3(MP2)//B3-SBK theory showed a mean deviation and deviation standard equal to 3.7 kcal mol−1 and 6.0 kcal mol−1, respectively. The G2 theory (mean deviation = 3.1 kcal mol−1 and standard deviation = 4.9 kcal mol−1) presented a lower error and standard deviation, but at a significantly higher computational cost. For a more complete evaluation, as a secondary part of the work, it also used different functionals B3LYP, M06-2X, WB97XD, and MP2 method with four different basis sets 6-311G(d,p), LANL2DZ, jorge-ADZP, and CEP-31G(d). The best density functional/basis set combination was obtained with M06-2X/CEP-31G(d) among the three mentioned functionals. However, the produced mean deviation is significant and equal to 17.3 kcal mol−1, with a standard deviation equal to 23.0 kcal mol−1. The 6-311G(d,p) basis achieved the best performance with the MP2 method, generating an equally significant mean deviation of 12.8 kcal mol−1 with a standard deviation equal to 18.7 kcal mol−1.

Similar content being viewed by others
References
Tatsuo K (2015) Iodine chemistry and applications. Wiley, Hoboken
Santos VM, Afonso JC (2013) QNEsc 35:297–298
Santos VM, Afonso JC (2012) Quim Nova 35:398–402
Leal RC, Custodio R (2019) Comput Theor Chem 1149:1–7
Baboul AG, Curtiss LA, Redfern PC, Raghavachari K (1999) J Chem Phys 110:7650–7657
Rocha CMR, Pereira DH, Morgon NH, Custodio R (2013) J Chem Phys 139:184108–184119
Silva CS (2020) Theor Chem Acc 139:135–142
Silva CS, Custodio R (2018) Theor Chem Acc 137:24–32
Curtiss LA, Redfern PC, Raghavachari K (2007) J Chem Phys 126:084108–084119
Silva CS, Custodio R (2015) Rev Proc Q 9:66–67
Silva CS, Custodio R (2019) J Phys Chem A 123:8314–8320
Silva CS, Pereira DH, Custodio R (2016) J Chem Phys 144:204118–204126
Leal RC, Pereira DH, Custodio R (2018) Comput Theor Chem 1123:161–168
Rocha CMR, Rodrigues JAR, Moran PJS, Custodio R (2014) J Mol Model 20:2524–2531
Filho SQA, Costa AMF, Ribeiro IHS, Custodio R, Pereira DH (2019) Comput Theor Chem 1166:112589–112595
Curtiss LA, Raghavachari K, Trucks GW, Pople JA (1991) J Chem Phys 94:7221–7230
Glukhovtsev MN, Pross A, McGrath MP, Radom L (1995) J Chem Phys 103:1878–1885
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 (Revision A.02)
Curtiss LA, Raghavachari K, Redfern PC, Pople JA (1997) J Chem Phys 106:1063–1079
Luo YR (2007) Comprehensive handbook of chemical bond energies. CRC Press, Taylor and Francis Group, Boca Raton
Wagman DD, Evans WH, Parker VB, Schumm RH, Halow I, Bailey SM, Churney KL, Nuttall RL (1982) The NBS tables of chemical thermodynamic properties selected values for inorganic and C1 C2 organic substance in SI units. J Phys Chem Ref Data 11:1–392
Stevens WJ, Basch H, Krauss M (1984) J Chem Phys 81:6026–6033
Stevens WJ, Krauss M, Basch H, Jasien PG (1992) Can J Chem 70:612–630
Linstrom PJ, Mallard WG (2022) NIST CHEMISTRY WebBook, NIST Standard Reference Database Number 69
Wadt WR, Hay PJ (1985) J Chem Phys 82:284–298
Bergner A, Dolg M, Küchle W, Stoll H, Preuss H (1993) Mol Phys 80:1431–1441
Peterson KA, Figgen D, Goll E, Stoll H, Dolg M (2003) J Chem Phys 119:11113–11123
Pople JA, Head-Gordon M, Fox DJ, Raghavachari K, Curtiss LA (1989) J Chem Phys 90:5622–5629
Becke AD (1988) Phys Rev A 38:3098–3100
Lee C, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Chai JD, Head-Gorgon M (2008) Phys Chem Chem Phys 10:6615–6620
Møller C, Plesset MS (1934) Phys Rev 46:618–622
McLean AD, Chandler GS (1980) J Chem Phys 72:5639–5648
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
Dunning TH Jr, Hay PJ (1977) Gaussian basis sets for molecular calculations. In: Schaefer HF (ed) Methods of Electronic Structure Theory. Modern Theoretical Chemistry, Springer Boston, Massachusetts, pp 1–27
Neto AC, Muniz EP, Centoducatte R, Jorge FE (2005) J Mol Struc 718:219–224
Oliveira PJP, Barros CL, Jorge FE, Neto AC, Campos M (2010) J Mol Struc 948:43–46
Ruscic B, Pinzon RE, Morton ML, Laszewski GV, Bittner S, Nijsure SG, Amin KA, Minkoff M, Wagner AF (2004) J Phys Chem A 108:9979–9997
Ruscic B, Pinzon RE, Laszewski GV, Kodeboyina D, Burcat A, Leahy D, Montoya D, Wagner AF (2005) J Phys Conf Ser 16:561–570
Settle JL, Jeffes JHE, O’Hare PAG, Hubbard WN (1976) J Inorg Nucl Chem 28:135–140
Curtiss LA, Raghavachari K, Redfern PC, Pople JA (2000) J Chem Phys 112:7374–7383
Curtiss LA, Redfern PC, Raghavachari K (2007) J Chem Phys 127:124105–124112
Sookhaki E, Namazian M (2021) J Mol Graphics Modell 108:107985–107991
Richard L, Gaona X (2011) Geochem Cosmochim Acta 75:7304–7310
Bodi A, Shuman NS, Baer T (2009) Phys Chem Chem Phys 11:11013–11021
Lago AF, Kercher JP, BÖdi A, Sztáray B, Miller B, Wurzelmann D, Baer T (2005) J Phys Chem 109:1802–1809
Dávalos JZ, Notario R, Cuevas CA, Oliva JM, Saiz-Lopez A (2017) Comput Theor Chem 1099:36–44
Zherikova KV, Verevkin SP (2019) J Therm Anal Calorim 138:4045–4059
Acknowledgements
The authors wish to thank Dr. Rogério Custodio for the help in the discussion and suggestions. Ysa Beatriz Dantas Marinho and Maria Andreizi Monteiro de Andrade thank the Institutional Research Support Program and the Research and Innovation Dean (PROPI) from IFRN for the research grants granted in the Research Notices nº 01/2019 (1st Call)—PROPI/RE/IFRN—Development of Research and Innovation, Projects nº 04/2020—PROPI/RE/IFRN—Research and Innovation Projects with Support, respectively. The authors would like to acknowledge the National Center of High-Performance Computing in Ceará (CENAPAD-UFC) for access to their computational facilities.
Author information
Authors and Affiliations
Contributions
Leal, R. C. and Sousa, I. L. analysis of data and writing. Marinho, Y. B. D. and Andrade, M. A. M. performed the calculations.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection XXI − Brazilian Symposium of Theoretical Chemistry (SBQT2021)
Appendix
Appendix
Table 4
Table 5
Table 6
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leal, R.C., Marinho, Y.B.D., de Andrade, M.A.M. et al. Determination of the standard enthalpy of formation of iodine compounds through the G2 and G3(MP2)//B3-SBK theories. J Mol Model 28, 246 (2022). https://doi.org/10.1007/s00894-022-05243-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05243-3